Question
Question: Calculate the force acting between two protons separated at a distance 20cm in free space....
Calculate the force acting between two protons separated at a distance 20cm in free space.
Solution
The two protons will repel each because like charges repel each other. Use Coulomb's formula for finding the force between the two protons. Note that the magnitude of force exerted by proton 1 on proton 2 is equal to the magnitude of force exerted by proton 2 on proton 1.
Formula used:
F=r2Kq1q2
Complete answer:
Let us first understand the force of interaction between two protons. The force between the two protons is an electrostatic force of repulsion.
Electrostatic force is a force that is produced between charges. Suppose there are two charges separated by some distance. The charge 1 will exert an electrostatic force on the charge 2 and similarly the charge 2 will exert an electrostatic force on the charge 1. Both the forces are of equal magnitudes but are in opposite directions along the line joining the two charges.
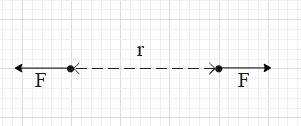
If both the charges are positive or both the charges are negative, then the two charges will repel each other.
We know that protons are positively charged. Therefore, the two will repel each other.
The magnitude of the electrostatic force between two charges q1, q2, which are separated by a distance r is given as F=r2Kq1q2 ….. (i),
where K is a proportionality constant whose value is 9×109Nm2C−2 in free space.
The charge on the proton is 1.6×10−19C. Therefore, q1=q2=1.6×10−19C
It is given that the protons are separated by a distance of 20cm. Therefore, r=20cm=0.2m.
Substitute the values in (i).
⇒F=0.229×109×1.6×10−19×1.6×10−19=5.76×10−27N.
Note:
If the charges were of opposite nature, i.e. one positive and one negative then the two charges will attract each other. Consider the two charges to be a proton and an electron separated by the same distance. The magnitude of charge on an electron is equal to the charge on a proton. Therefore, the two charges will attract each other with the same magnitude of force that was in the case of two protons.
