Question
Question: Calculate the enthalpy change of the following reaction $H_2C = CH_2(g) + H_2(g) \rightarrow H_3C -...
Calculate the enthalpy change of the following reaction
H2C=CH2(g)+H2(g)→H3C−CH3(g)
The bond energy of C - H, C - C, C = C, H - H are 414, 347, 615 and 453 KJ mol−1 respectively.
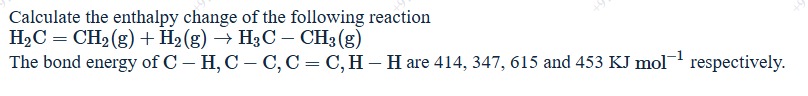
Answer
The enthalpy change of the reaction is -107 KJ/mol.
Explanation
Solution
The enthalpy change of the reaction can be calculated as the sum of bond energies of bonds broken in reactants minus the sum of bond energies of bonds formed in products.
Bonds broken: 1 C=C, 4 C-H, 1 H-H. Total energy for breaking bonds = 615+4(414)+453=2724 KJ/mol.
Bonds formed: 1 C-C, 6 C-H. Total energy for forming bonds = 347+6(414)=2831 KJ/mol.
Enthalpy change ΔH=2724−2831=−107 KJ/mol.
