Question
Question: Calculate the degree of hydrolysis and pH of 0.1 M sodium acetate solution. The hydrolysis constant ...
Calculate the degree of hydrolysis and pH of 0.1 M sodium acetate solution. The hydrolysis constant of sodium acetate is5.6x10−10.
Solution
Sodium acetate is the sodium salt form of acetic acid. Write down the balanced chemical reaction of sodium acetate with water for hydrolysis. With the help of the degree of hydrolysis, calculate the concentration of hydrogen ions, and calculate the pH. Hydrolysis constant is equal to the ratio of concentration of the product and concentration of the reactant. pH is the negative logarithm of the concentration of the hydrogen ions in the solution
Complete step by step solution:
The degree of hydrolysis of salt is defined as the fraction or percentage of the total salt which is hydrolyses, i.e.,
The equilibrium reaction of hydrolysis of sodium acetate or for convenience hydrolysis of acetate:
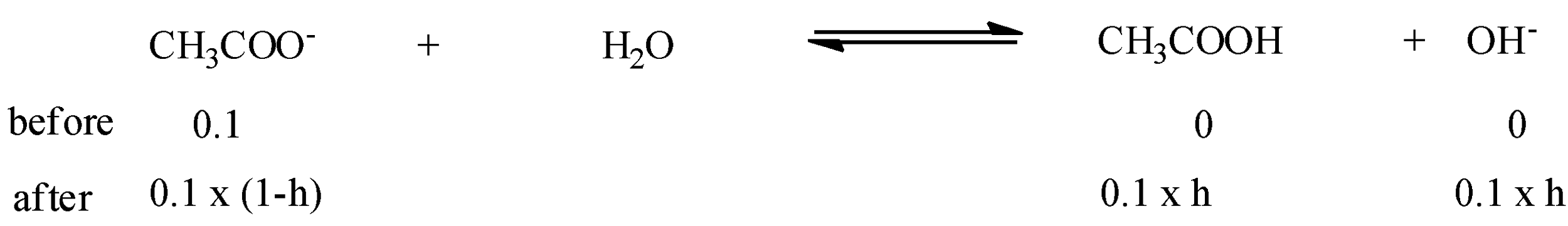
Acetate forms acetic acid and hydroxyl ions.
Hence, with this chemical equation, we can formulate,
Kh=[CH3COO−][CH3COOH][OH−]
Hydrolysis constant is equal to the ratio of the concentration of the product and the concentration of the reactant.
Now, putting the values of concentration after the equilibrium,
=0.1(1−h)(0.1 x h)(0.1 x h)
Since the acetate is a weak base hence the h is small that it can be ignored:
=0.1(0.1 x h)(0.1 x h)
Kh=5.6x10−10
Putting the values, we get, 5.6 x 10−10=0.1 x h2
h2=0.15.6x10−10=56x10−10
h=56x10−10=7.48x10−5
Degree of hydrolysis is 7.48x10−5
For finding the pH, the concentration of hydrogen ions is calculated:
The concentration of hydroxyl ions will be:
[OH−]=ch=0.1x7.48x10−5=7.48x10−6M
Now, the concentration of hydrogen ions can be calculated by: [H+]=[OH−]Kw
Kwis the ionic product of water, which is 10−14
[H+]=7.48x10−610−14=1.33x10−9M
Hence, the concentration of hydrogen ion is 1.33x10−9M
Now, pH is the negative logarithm of the concentration of the hydrogen ions in the solution.
pH=−log[H+]=−log(1.33x10−9)=8.88
The pH of the solution is 8.88.
Hence the degree of hydrolysis of 0.1 M of sodium acetate is7.48x10−5 and pH of the solution is 8.88.
Note: For weak acid or weak base the value of h is very small, so it can be ignored but for strong acid or strong base, the value of h cannot be ignored. pH can be calculated directly by applying the formula pH=7+21[pKa+logc].
