Question
Question: Calculate standard internal energy change for $OF_{2(g)} + H_2O_{(g)} \rightarrow 2HF_{(g)} + O_{2(...
Calculate standard internal energy change for
OF2(g)+H2O(g)→2HF(g)+O2(g) at 300 K, if
ΔfH∘ of OF2(g), H2O(g) and HF(g) are 20, -250 and -270 kJ mol−1 respectively.
[R = 8.314 J K−1 mol−1]
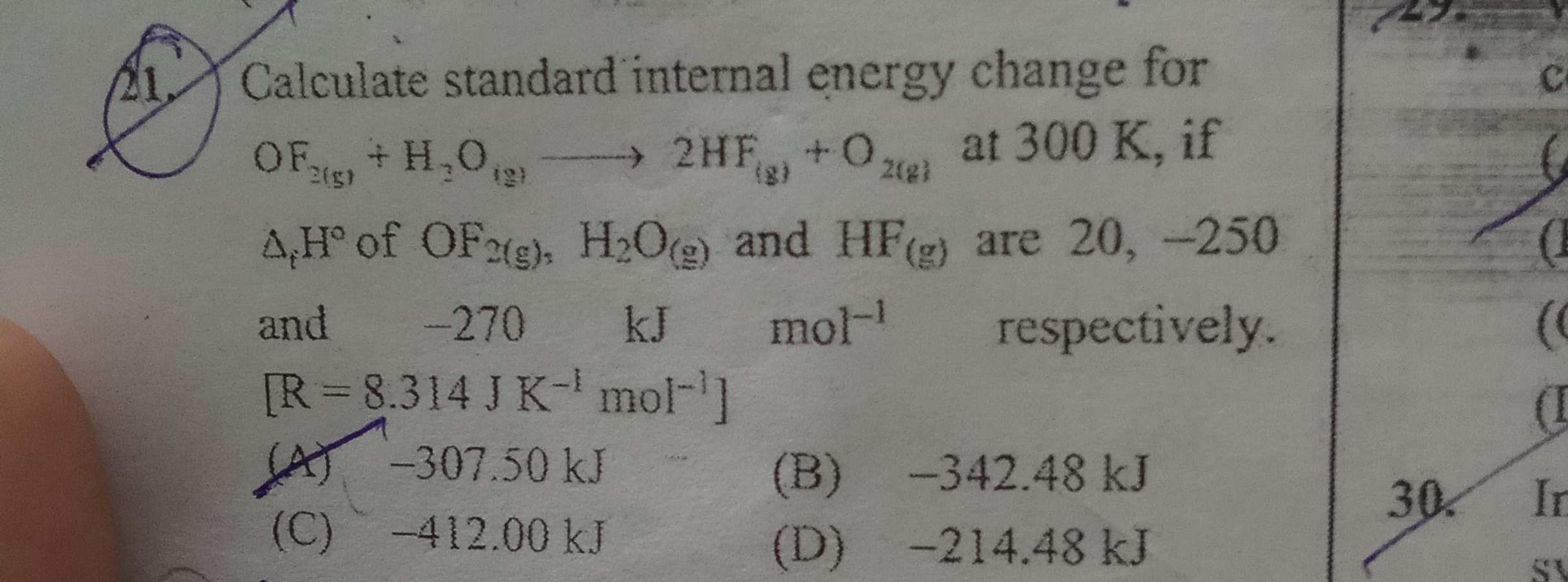
-307.50 kJ
-342.48 kJ
-412.00 kJ
-214.48 kJ
The closest answer is -307.50 kJ, but the calculated value is approximately -312.50 kJ.
Solution
To calculate the standard internal energy change (ΔU∘) for the given reaction:
-
Calculate the standard enthalpy change (ΔH∘) for the reaction:
ΔH∘=∑ΔfH∘(products)−∑ΔfH∘(reactants)
Given:
- ΔfH∘(OF2(g))=20kJ/mol
- ΔfH∘(H2O(g))=−250kJ/mol
- ΔfH∘(HF(g))=−270kJ/mol
- ΔfH∘(O2(g))=0kJ/mol (since it's an element in its standard state)
ΔH∘=[2×(−270)+0]−[20+(−250)]=−540−(−230)=−310kJ
-
Relate ΔU∘ and ΔH∘ using the formula:
ΔH∘=ΔU∘+ΔnRT where:
- Δn= (moles of gaseous products) - (moles of gaseous reactants)
- R=8.314J/mol\cdotpK=0.008314kJ/mol\cdotpK
- T=300K
-
Calculate Δn:
Δn=(2molHF+1molO2)−(1molOF2+1molH2O)=3−2=1
-
Calculate ΔnRT:
ΔnRT=1×0.008314kJ/mol\cdotpK×300K=2.4942kJ
-
Calculate ΔU∘:
ΔU∘=ΔH∘−ΔnRT=−310kJ−2.4942kJ=−312.4942kJ≈−312.50kJ
Therefore, the standard internal energy change for the reaction is approximately -312.50 kJ.
