Question
Question: Calculate packing efficiency in simple cubic lattice....
Calculate packing efficiency in simple cubic lattice.
Solution
Packing efficiency is a fraction of the unit cell actually occupied by spheres. It is the percentage of space covered by atoms to the actual space of the unit cell. Simple cubic is a type of primitive unit cell.
Formula used = (volume of cubevolume of spheres)×100
Complete step by step answer:
We know packing efficiency is the measure of how closely spheres are bound in a crystal lattice. Simple cubic lattice is a type of unit cell in which atoms are present only at corners (also called primitive cells).
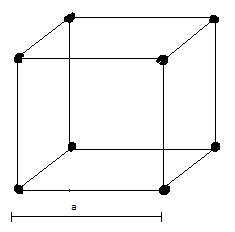
Above picture shows a simple cubic lattice with edge length a. Dark points refer to the centre of atoms.
Each atom in the corner is shared by eight unit cells. This means part of one atom present in one sphere is 81 as it is shared by eight unit cells. There are eight corners of cube, therefore
Number of atoms=81×8=1
There is one atom per unit cell in a simple cubic lattice.
Formula to calculate packing efficiency is
Packing efficiency=(volume of cubevolume of spheres)×100
In the lattice atom is represented as a sphere so volume of sphere in formula is equivalent to volume of atom.
On one edge there will be two atoms and both atoms will touch each other so if we take the radius of the sphere to be r then edge length a will be equal to 2r (as there are two spheres with their centre at corner of cube).
a=2r
volume of sphere=34Πr3
volume of cube=side3=a3
As written above a=2r
So, volume of cube=(2r)3=8r3
Packing efficiency=8r334Πr3×100
=6100Π
=52.35% Which is approximately equal to 52.4%
Note:
In simple cubic unit cell atoms are present only at corners. It is a primitive unit cell. In other unit cells atoms are present at corners as well as at other positions. In body centered cubic lattice atoms are present at corners as well as at the centre of the cube, in face centered cubic system atoms are present at corners as well as at the center of each face of the cube.
