Question
Question: Calculate mass of a divalent metal produced at cathode by passing 5 amp current through its salt sol...
Calculate mass of a divalent metal produced at cathode by passing 5 amp current through its salt solution for 100 minute.
(Molar mass of metal = x)
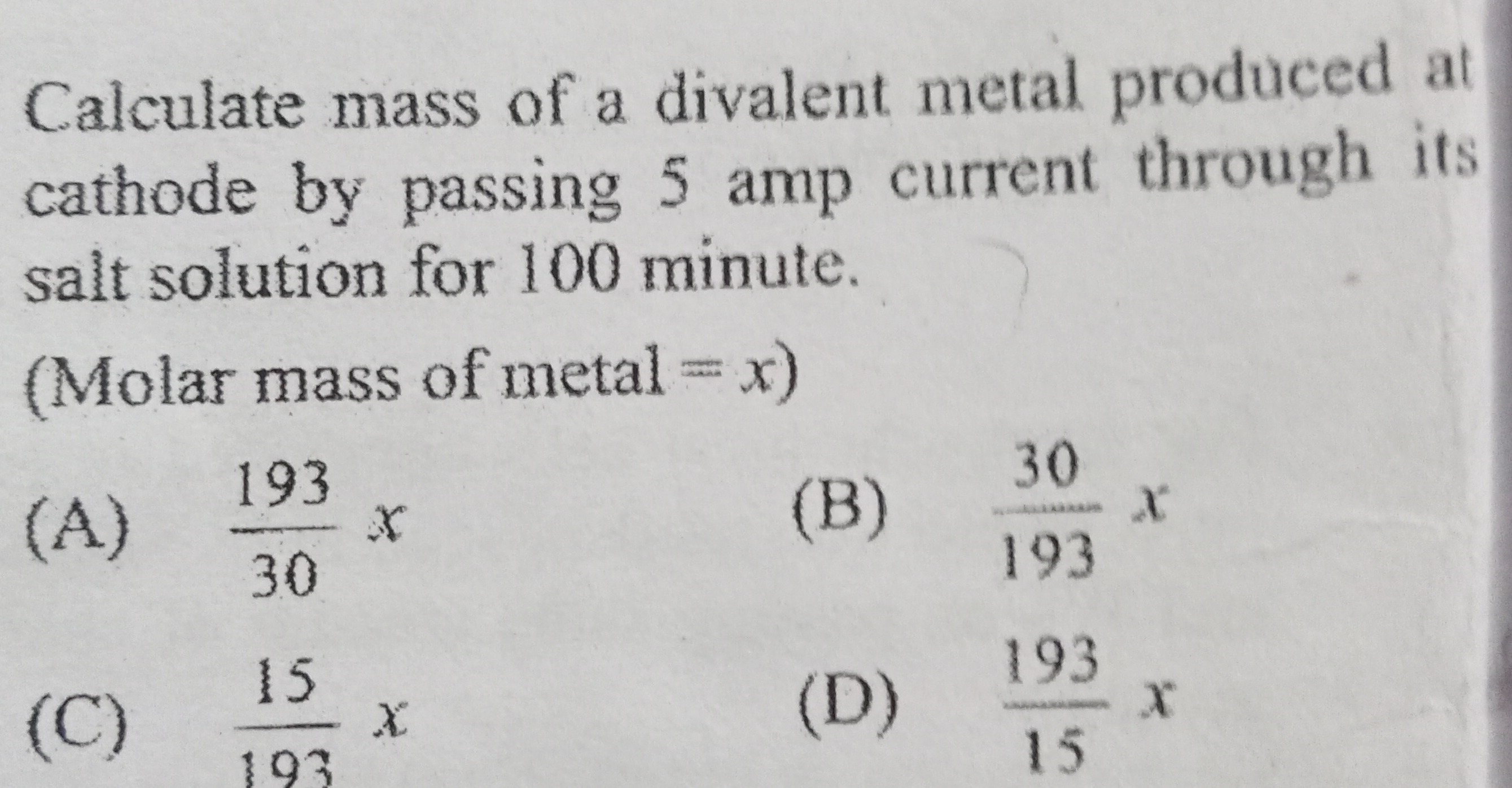
A
30193x
B
19330x
C
19315x
D
15193x
Answer
19330x
Explanation
Solution
Solution:
-
Calculate total charge (Q):
Time = 100 minutes = 6000 s
Q=I×t=5 A×6000 s=30000 C
-
Determine moles of electrons transferred:
For a divalent metal:
M+2+2e−→M
Moles of electrons = Q/F=30000/96500
Moles of metal deposited = (Q/F)/2=30000/(2×96500)=30000/193000=30/193
-
Calculate mass deposited:
Mass = (Moles of metal) × (Molar mass) = (30/193)×x
Thus, the mass of the metal produced is 19330x.
