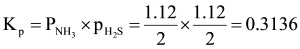Question
Question: Calculate <img src="https://cdn.pureessence.tech/canvas_547.png?top_left_x=1200&top_left_y=1179&widt...
Calculate  for the equilibrium,
for the equilibrium,
 NH3( g)+H2 S(g)
NH3( g)+H2 S(g)
If the total pressure inside the reaction vessel is 1.12atm at 105∘C .
A
0.56
B
1.25
C
0.31
D
0.63
Answer
0.31
Explanation
Solution
:  NH3( g)+H2 S(g)
NH3( g)+H2 S(g)
Initial moles 1 0 0
At equilibrium (1 – x) x x
Total gaseous moles at equilibrium = x + x = 2x
We know 
But,
Partial pressure (P) = mole fraction × total pressure (P)

IInd method: Both NH3 and H2 S have same number of moles at equilibrium so have same mole fraction and thus equal partial pressures.
i.e.,