Question
Question: Calculate heat of combustion of ethene from bond energy data: C=C, C-H, O=O...
Calculate heat of combustion of ethene from bond energy data:
C=C, C-H, O=O, C=O, O-H
BE (kJmol - 1): 619, 414, 499, 724, 460
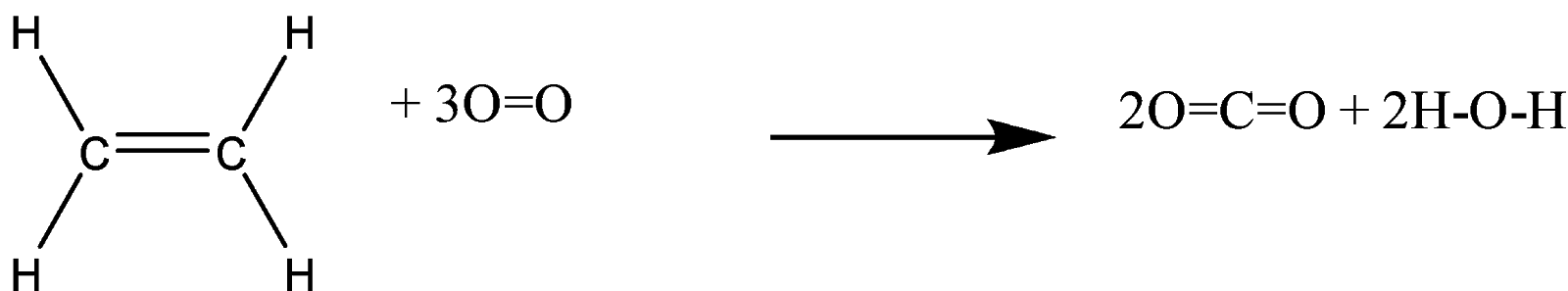
Solution
We have to calculate the heat of combustion for the given reaction of ethene. Look at the given data in the question. The formula used to calculate the heat combustion can be written as:
Heat of combustion = Bond energy of reactants – Bond energy of products
Complete step by step answer:
-Let us discuss the heat of combustion, it is also known as energy value.
-We can define the heat of combustion as the energy released when a substance will undergo combustion.
-We can see that in the given question, ethene undergoes combustion, and leads to the formation of carbon-dioxide, and water.
-So, we can say that in the above reaction, the reactants are ethene, and oxygen; and the products are carbon-dioxide, and water.
-Here, heat of combustion = bond energy of reactants – bond energy of products.
-First, let us calculate the bond energy of reactants, then
-Bond energy of reactants =Sum of bond energies of bonds present in the reactants
-Bond energy of reactants = 4 (C-H) + (C=C) + 3(O=O)
-Bond energy = 4 (414) + 619 + 3 (499) = 3772 kJ/mol
-Second, we will calculate the bond energy of products
-Bond energy of products = Sum of bond energies of bonds present in the products
-Bond energy of products = 4 (C=O) + 4 (O-H)
-Bond energy = 4 (724) + 4 (460) = 4736 kJ/mol
Thus, we can substitute the values, it can be written as:
-Heat of combustion = 3772 kJ/mol – 4736 kJ/mol
-Heat of combustion = -964 kJ/mol
In the last, we can say that the heat of combustion of ethene from the given data is -964 kJ/mol.
Note: Don’t get confused while calculating the heat of combustion for the given molecule. In this question, we could clearly see the bonds present in the reactants, and the products. We have substituted the C=O 4 times because we have attained 2 molecules of carbon-dioxide in the product. We know that one molecule of carbon-dioxide contains two C=O bonds. So, we have multiplied the value with 4.
