Question
Question: Calculate frequency of a photon having an energy of 2 electron volt....
Calculate frequency of a photon having an energy of 2 electron volt.
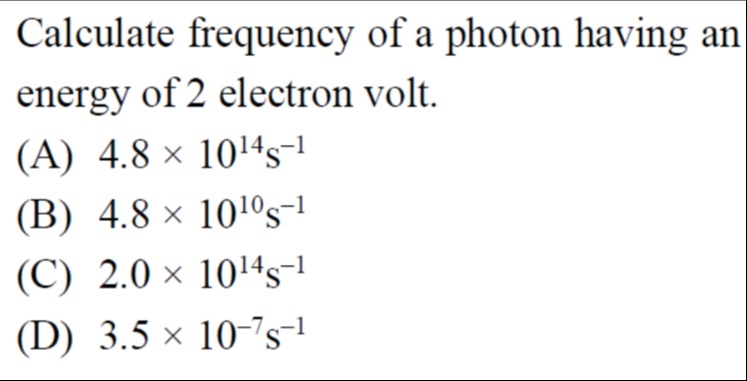
A
4.8 × 1014s-1
B
4.8 × 1010s-1
C
2.0 × 1014s-1
D
3.5 × 10-7s-1
Answer
4.8 × 1014s-1
Explanation
Solution
The energy (E) of a photon is related to its frequency (ν) by Planck's equation: E=hν. To find the frequency, we rearrange the formula: ν=hE. Given energy E=2 electron volts (eV). We convert this to Joules (J) using the conversion factor 1 eV=1.602×10−19 J: E=2 eV×(1.602×10−19 J/eV)=3.204×10−19 J. Planck's constant h≈6.626×10−34 J.s. Now, we calculate the frequency: ν=6.626×10−34 J.s3.204×10−19 J≈4.835×1014 s−1. This value is approximately 4.8×1014 s−1.
