Question
Question: Calculate ΔΗ for following reaction, at 25°C. $NH_2CN_{(g)} + \frac{3}{2}O_{2(g)} \longrightarrow N...
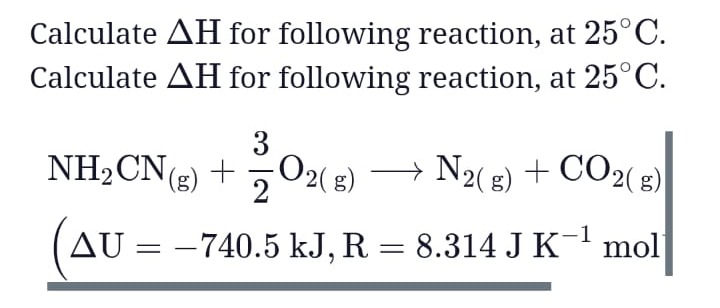
Calculate ΔΗ for following reaction, at 25°C.
NH2CN(g)+23O2(g)⟶N2(g)+CO2(g)
(ΔU=−740.5 kJ, R = 8.314 J K−1 mol)
Answer
ΔH ≈ -741.7 kJ
Explanation
Solution
Given the reaction:
NH2CN(g)+23O2(g)⟶N2(g)+CO2(g)
-
Count moles of gases:
- Reactants:
- NH2CN: 1 mole
- O2: 1.5 moles
- Total = 2.5 moles
- Products:
- N2: 1 mole
- CO2: 1 mole
- Total = 2 moles
Change in moles:
Δn=2−2.5=−0.5 moles
- Reactants:
-
Use the relation between ΔH and ΔU:
ΔH=ΔU+Δ(PV)
For ideal gases,
Δ(PV)=ΔnRT
Given:
- ΔU=−740.5kJ
- T=298K (25°C)
- R=8.314JK−1mol−1=0.008314kJK−1mol−1
-
Calculation:
Δ(PV)=−0.5×0.008314K molkJ×298K≈−1.2385kJ
Thus,
ΔH=−740.5kJ−1.2385kJ≈−741.74kJ
Answer:
ΔH≈−741.7kJ
