Question
Question: Calcium butanoate on heating followed by 1,2-ethanediol in the presence of catalytic amount of acid,...
Calcium butanoate on heating followed by 1,2-ethanediol in the presence of catalytic amount of acid, produces a major product which is:
A. 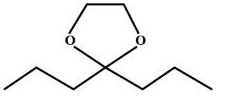
B. 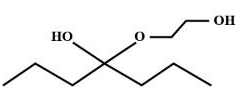
C. 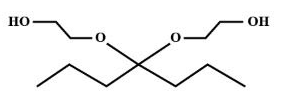
D. 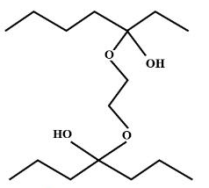
Solution
Hint: When ever organic reaction takes place, two types of product can be formed. One is major and the other is minor. Major product is that product which is more likely to be formed in a reaction. Minor products are those which are less likely to be formed in a chemical reaction.
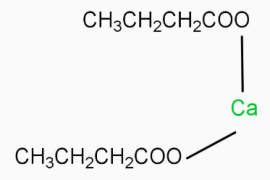 is the chemical formula for calcium butanoate.
is the chemical formula for calcium butanoate.
Complete step by step solution:
- 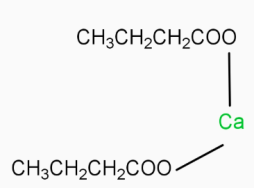 ( Calcium butanoate ) Δ
( Calcium butanoate ) Δ 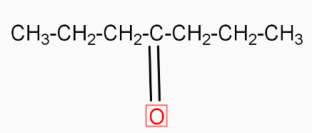 ( 4-heptanone ) Calcium butanoate when heated eliminates CaCO3( calcium carbonate ). The product formed in this reaction is 4-heptanone
( 4-heptanone ) Calcium butanoate when heated eliminates CaCO3( calcium carbonate ). The product formed in this reaction is 4-heptanone
- After heating it is given that it is reacted with 1,2-ethanediol in the presence of catalytic amount of acid. Acid means in the presence of H+.
- 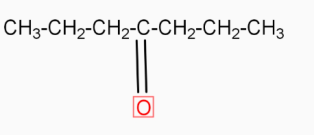 ( 4-heptanone ) in the presence of
( 4-heptanone ) in the presence of 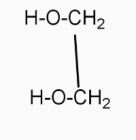 (1,2-ethanediol ) and →H+
(1,2-ethanediol ) and →H+ 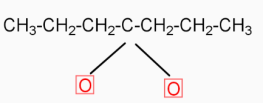 (2,2-dipropyl-1,3-dioxolane ).
(2,2-dipropyl-1,3-dioxolane ).
So, we can say that option A is correct.
Additional Information:
- Butyrate or butanoate the name is used for the conjugate base of butyric acid.
- Butyrate is used as food for cells lining the mammalian colon.
- In the human body it is produced by our gut bacteria and supports digestive health and disease prevention.
Note: In the above reaction we use acid as a catalyst, the use of acid catalyst for organic chemical reaction is they serve as a source of protons. In the above reaction H+ combines with the OH group to form H2O which is eliminate to form the final product.
