Question
Question: Calc the $eq^m$ conc. of $PCl_3$ in 10L rigid vess of 1 mole of $PCl_5$ is ______ to attain the foll...
Calc the eqm conc. of PCl3 in 10L rigid vess of 1 mole of PCl5 is ______ to attain the following reactn.
PCl5(g)⇌PCl3+Cl3(g)
(i) if kc=10−5 at temp T1 if kc=10+5 at temp T2 if kc=10−2 at temp T3
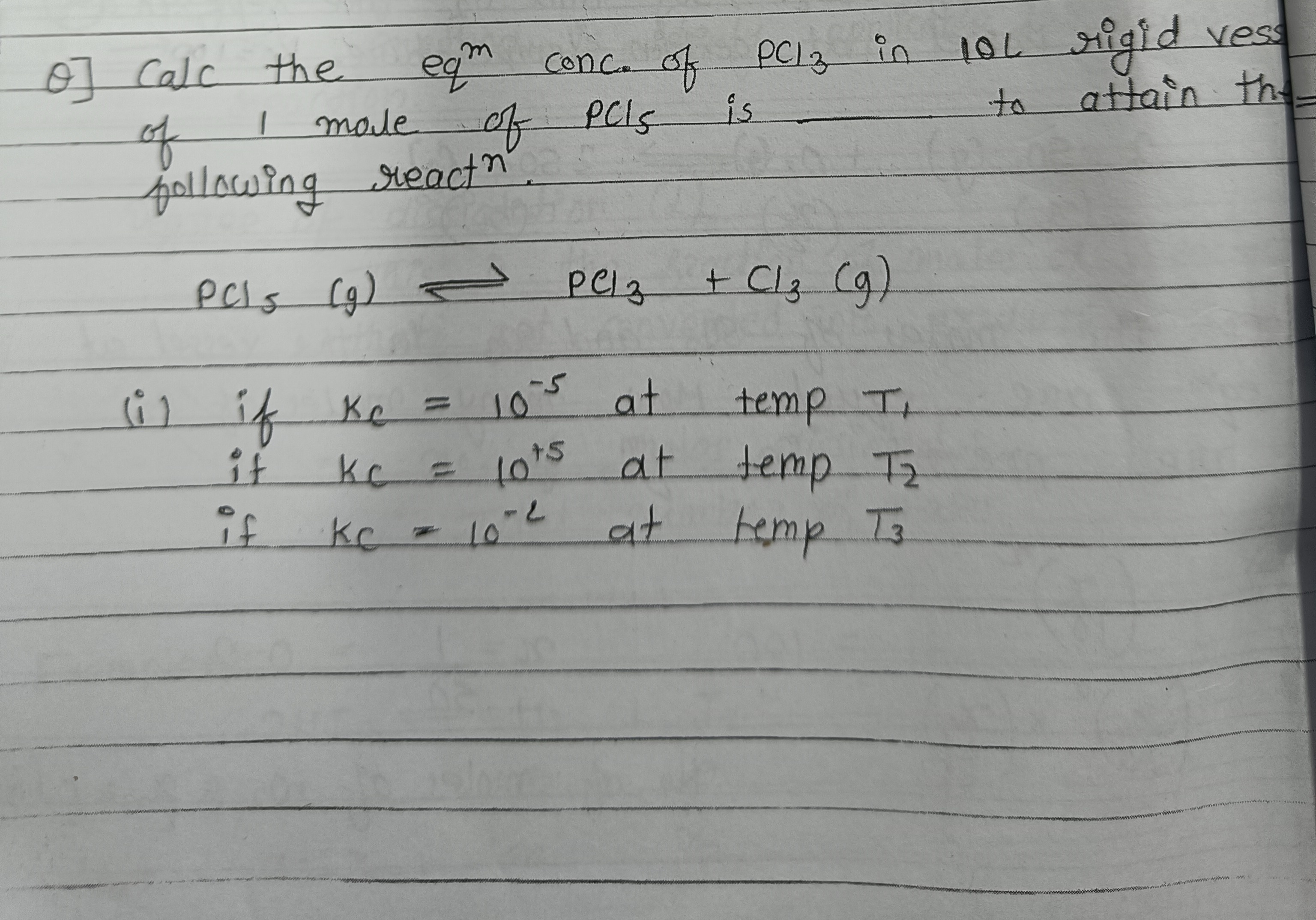
(i) 10^{-3} M, (ii) 0.1 M, (iii) 0.027 M
Solution
The reaction is PCl5(g)⇌PCl3(g)+Cl2(g).
Initial moles: PCl5=1, PCl3=0, Cl2=0 in a 10L vessel. Let x be the moles of PCl5 that dissociate at equilibrium.
Equilibrium moles: PCl5=1−x, PCl3=x, Cl2=x.
Equilibrium concentrations (Volume = 10L): [PCl5]=101−x M [PCl3]=10x M [Cl2]=10x M
The equilibrium constant Kc is given by: Kc=[PCl5][PCl3][Cl2]=(101−x)(10x)(10x)=10(1−x)x2
We need to solve for x for each given value of Kc and then calculate [PCl3]=10x. The equation is Kc=10(1−x)x2, which can be rearranged to x2+10Kcx−10Kc=0. The valid solution for x must be in the range 0≤x≤1.
(i) If Kc=10−5: The equation is x2+10(10−5)x−10(10−5)=0⟹x2+10−4x−10−4=0. Since Kc is very small, the reaction extent x will be small (x≪1). We can approximate 1−x≈1. Kc≈10x2 10−5≈10x2⟹x2≈10−4⟹x≈10−4=10−2. Since x=10−2=0.01 is much smaller than 1, the approximation is valid. Equilibrium concentration of PCl3 is [PCl3]=10x≈1010−2=10−3 M.
(ii) If Kc=10+5: The equation is x2+10(105)x−10(105)=0⟹x2+106x−106=0. Since Kc is very large, the reaction proceeds almost to completion, so x is close to 1. Let x=1−y, where y is small (y≪1). Kc=10y(1−y)2. Since y is small, (1−y)2≈12=1. 105≈10y1⟹106y≈1⟹y≈10−6. So, x=1−y≈1−10−6=0.999999. Equilibrium concentration of PCl3 is [PCl3]=10x≈101−10−6=0.1−10−7 M. This is very close to 0.1 M.
(iii) If Kc=10−2: The equation is x2+10(10−2)x−10(10−2)=0⟹x2+0.1x−0.1=0. Using the quadratic formula: x=2−0.1±(0.1)2−4(1)(−0.1)=2−0.1±0.01+0.4=2−0.1±0.41. Since x>0, we take the positive root: x=2−0.1+0.41. 0.41≈0.6403. x≈2−0.1+0.6403=20.5403=0.27015. Equilibrium concentration of PCl3 is [PCl3]=10x≈100.27015=0.027015 M.
Summary of equilibrium concentrations of PCl3: (i) Kc=10−5: [PCl3]≈10−3 M (ii) Kc=10+5: [PCl3]≈0.1 M (iii) Kc=10−2: [PCl3]≈0.027 M
