Question
Question: \(Ca{C_2}\xrightarrow{{{H_2}O}}X \to Y;YIS:\) A.
B.
C.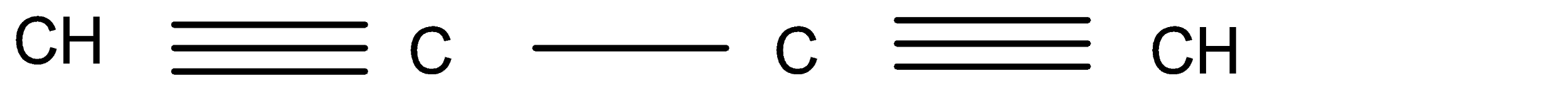
D.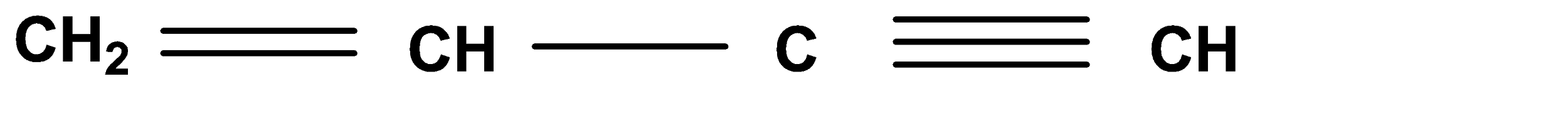
Solution
Calcium carbide reacts with water to produce calcium hydroxide and acetylene. Then calcium hydroxide and acetylene react with each other and give us the final product. This reaction completed in two steps to get final products. Calcium carbide is not volatile and soluble in any known solvent; it reacts with water to give yield.
Complete step by step answer:
Calcium carbide reacts with water, this reaction was the basis of the industrial production of acetylene. Calcium carbides react with water at high temperature and form acetylene and calcium hydroxide.
This process has taken two step:
1.Reaction between calcium carbide and water, which gives us Product-X.
2.Reaction between acetylene and calcium hydroxide, which gives us Product-Y.
Now we understand step-1:
CaC2+H2O→C2H2+Ca(OH)2
In the above reaction, Calcium carbide reacts with water and gives yield of acetylene and calcium hydroxide, which is our Product-X.
Now, we find out about Product-Y.
The reaction between acetylene and calcium hydroxide given as below,

In the second step we get the product in red colour. The reaction occurs in the presence of ammonium hydroxide and copper chloride. Here copper acetylide is produced which is red in colour and it is considered as a confirmed test for terminal alkynes.
Hence, Option (A) is the correct answer.
Note: Ethyne and calcium hydroxide are formed when calcium carbide is treated with water in the molar ratio of 2:1 . Ethyne and calcium hydroxide react to produce copper acetylide. In this reaction we remember that the reaction occurs in the presence of copper chloride and ammonium hydroxide. As we know that calcium carbide is mainly used for the production of acetylene and calcium cyanamide.
