Question
Question: \(C{O_2}\) is gas while \(Si{O_2}\) is solid because: A: \(C{O_2}\) is a linear molecule, while \(...
CO2 is gas while SiO2 is solid because:
A: CO2 is a linear molecule, while SiO2 is agular.
B: van der waals’ forces are very strong in SiO2.
C: CO2 is covalent, while SiO2 is ionic.
D: Si cannot form stable bonds with O, hence Si has to form a 3D lattice.
Solution
Carbon and silicon both belong to the same group in the periodic table. This means these atoms have similar properties. These atoms have a tendency to show catenation. Carbon shows catenation to the maximum extent.
Complete step by step answer:
Carbon and silicon both belong to the same group in the periodic table. This means these atoms have similar properties. These atoms have a tendency to show catenation. Catenation is the property of an element with which it can form a long chain by linking with other atoms of the same element. In CO2 there is a double bond between carbon and oxygen atoms. Carbon has a tendency to make bonds with oxygen but silicon cannot make bonds with oxygen due to which it forms a giant molecular network with oxygen. Structure of this molecule is as follows:
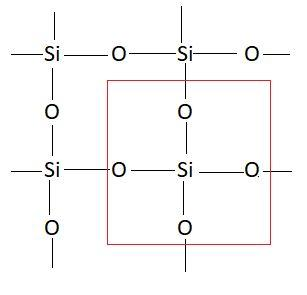
Due to this the bonding in this molecule is very strong and hence it exists as solid. This means due to the inability of silicon atoms to make bonds with oxygen SiO2 make a giant network. In this network bonding is strong and hence this exists as solid. So, the correct answer is option D that is Si cannot form stable bonds with O, hence Si has to form a 3D lattice.
So, the correct answer is Option D.
Note:
Catenation is the property of an element with which it makes bonds with other atoms of the same element. Carbon shows maximum catenation. Size of carbon is small due to which the hold of the nucleus of the shared pair of electrons is more and it is able to make long chains.
