Question
Question: Number of electrons present in 3.6 mg of NH$_{4}^{+}$ are : (N$_{A}$ = 6x10$^{23}$)...
Number of electrons present in 3.6 mg of NH4+ are : (NA = 6x1023)
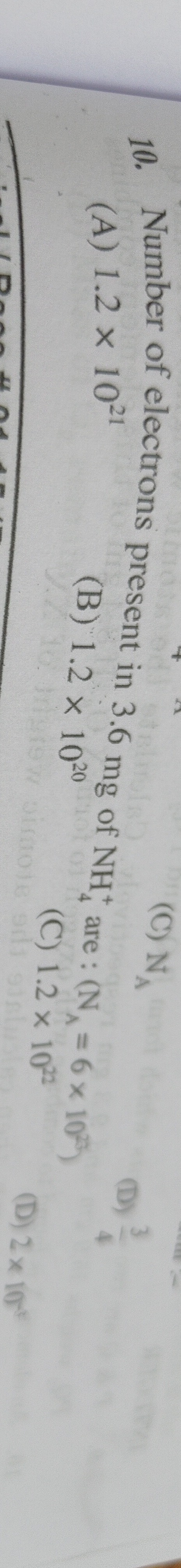
A
1.2 x 1021
B
1.2 x 1020
C
1.2 x 1022
D
43
E
2 x 1023
Answer
1.2 x 1021
Explanation
Solution
Here's a step-by-step explanation to calculate the number of electrons in 3.6 mg of NH4+:
-
Calculate the molar mass of NH₄⁺:
- Atomic mass of Nitrogen (N) = 14 g/mol
- Atomic mass of Hydrogen (H) = 1 g/mol
- Molar mass of NH₄⁺ = (1 × 14) + (4 × 1) = 14 + 4 = 18 g/mol
-
Calculate the number of moles of NH₄⁺:
- Given mass = 3.6 mg = 3.6 × 10⁻³ g
- Number of moles = Molar massMass
- Number of moles = 18 g/mol3.6×10−3 g=0.2×10−3 mol=2×10−4 mol
-
Calculate the number of NH₄⁺ ions:
- Number of ions = Number of moles × Avogadro's number (NA)
- Given NA = 6 × 10²³
- Number of ions = (2×10−4 mol)×(6×1023 ions/mol)
- Number of ions = 12×1019 ions=1.2×1020 ions
-
Calculate the number of electrons in one NH₄⁺ ion:
- Number of electrons in Nitrogen (N) = Atomic number of N = 7
- Number of electrons in Hydrogen (H) = Atomic number of H = 1
- Total electrons in a neutral NH₄ (hypothetical) = 7 (from N) + 4 × 1 (from 4H) = 11 electrons
- Since it is NH₄⁺, it has lost one electron (due to the +1 charge).
- Number of electrons in NH₄⁺ = 11 - 1 = 10 electrons
-
Calculate the total number of electrons:
- Total electrons = Number of NH₄⁺ ions × Number of electrons per ion
- Total electrons = (1.2×1020 ions)×(10 electrons/ion)
- Total electrons = 12×1020 electrons=1.2×1021 electrons
