Question
Question: \(C{{H}_{4}}\) has: a.) Linear Geometry b.) Bent geometry c.) Tetrahedral geometry d.) Pyram...
CH4 has:
a.) Linear Geometry
b.) Bent geometry
c.) Tetrahedral geometry
d.) Pyramidal geometry
Solution
Geometry of the molecule depends on two factors. One is hybridization and second is number of lone present in central atom. As in Carbon there will not be a lone pair for the given molecule, that’s why it's geometry will purely be decided on the basis of hybridization alone. In case of sp hybridization, a molecule will have linear geometry and in case of sp3 hybridization molecule will have tetrahedral geometry. Since Carbon doesn’t have any lone pair, therefore it can’t have bent or pyramidal geometry.
Complete Solution :
Electronic configuration for of Carbon:
1s22s22p2
- As carbon has to accommodate 4 hydrogen atoms, therefore it needs 4 half filled orbitals, so one 2s electron will be transferred into 2p orbital. Now new electronic configuration is:
1s22s12p3
Now it has one s orbital and 3 p orbitals as half filled orbitals and no lone pair in valence shell, therefore these 4 orbitals will take part in hybridization, so there will be clearly sp3 hybridization.
We know that molecule that has sp3 hybridization without any lone pair, always has tetrahedral geometry.
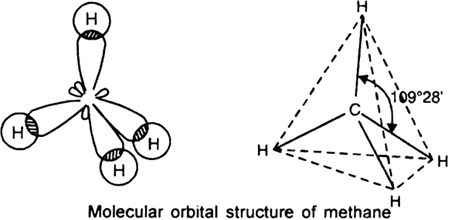
So CH4 has tetrahedral geometry.
So, the correct answer is “Option C”.
Additional Information:
In a tetrahedral molecular geometry, a central atom is located at the center with four substituents that are located at the corners of a tetrahedron. It has the bond angle of 109028′. It is a symmetrical shape.
Note: Methane is gas that is found in small quantities in Earth's atmosphere. Methane is a powerful greenhouse gas. Methane is flammable, and is used as a fuel worldwide. It can explode at concentrations between 5% (lower explosive limit) and 15% (upper explosive limit). It is relatively non-toxic gas. Its health effects are associated with being simple asphyxiate displacing oxygen in the lungs.
