Question
Question: \(C{H_3}Cl\) has more dipole moment than \(C{H_3}F\) because: (A) Electron affinity of chlorine is...
CH3Cl has more dipole moment than CH3F because:
(A) Electron affinity of chlorine is greater than that of fluorine
(B) The charge separation is larger in CH3Cl compared to CH3F
(C) The repulsion between the bond pairs and non – bonded pairs of electrons is greater in CH3Cl than CH3F
(D) Chlorine has higher electronegativity than fluorine
Solution
Dipole moment is the result of charge separation between positive and negative charges. Dipole moment is calculated by the product of charge and distance of separation.
μ=q×d
μ= dipole moment
q = charge
d = distance of separation
Its units are coulomb meter or debye.
Complete step by step answer: The structure of CH3Cl is:
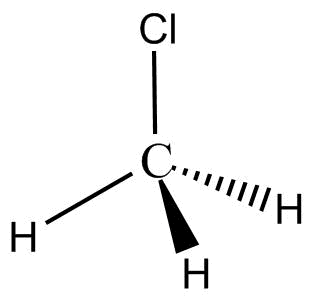
Its IUPAC name is methyl chloride. Its shape is tetrahedral. In CH3Cl carbon is the central atom three hydrogens and one chlorine is arranged around carbon. CH3Cl Molecule is a dipole molecule as carbon is more electronegative than hydrogen. Therefore, the shared pair of electrons move towards carbon and hydrogen obtain a partial positive charge.
In turn, chlorine is more electronegative than carbon thus it attracts the shared electrons and gets partial negative charge. Thus, the molecule has both partial positive and partial negative charge. The bond angle in CH3Cl is 109.5∘
According to the formula of dipole moment, it depends on both charge and distance between charges. Fluorine is more electronegative than chlorine, but C−Cl bond is larger than C−F bond is 178 and 139pm respectively.
Therefore, by considering both the points due to large distance of separation in CH3Cl as compared to CH3F, the dipole moment of CH3Cl is greater than CH3F.
Hence, the correct option is (B) the charge separation is larger in CH3Cl compared to CH3F.
Note: The dipole moment of a single bond in a polyatomic molecule is very different from the dipole moment in a molecule as a whole. It is a vector quantity and also can be zero.
