Question
Question: \(C{{H}_{3}}-CHC{{l}_{2}}\) on hydrolysis will give (A)- \(C{{H}_{3}}CHO\) (B)- \(C{{H}_{3}}COOH...
CH3−CHCl2 on hydrolysis will give
(A)- CH3CHO
(B)- CH3COOH
(C)- CH3CH2OH
(D)- CH3−O−CH3
Solution
In the hydrolysis of the given compound, the nucleophile from the water molecule, displaces the halogen atoms in the compound, in presence of an alkaline medium. Also, the halides are good leaving groups, thus producing a C-O double-bond in the carbonyl compound formed.
Complete answer:
Hydrolysis of the given gem-dihalide compound, that is, 1,1-dichloroethane, takes place in presence of basic medium. It undergoes nucleophilic substitution reactions.
The SN2 mechanism is followed, in which the nucleophile, that is, the hydroxide ion from the water molecule, attacks the partially electropositive carbon atom of the compound from the back.
At the same time, in the transition state, the chloride ion of the compound departs from the front because being a weak conjugate base, it is relatively stable in nature. Thus, acting as a good leaving group. A gem-halohydrin is formed.
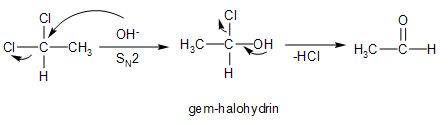
The remaining chloride group, also being a good leaving group departs from the compound, leading to the formation of carbonyl group. The product formed is acetaldehyde.
Therefore, CH3−CHCl2on hydrolysis will give option (A)- CH3CHO.
Additional information:
In the geminal dihalide, when both the halogens are present on the terminal carbon, it gives aldehyde on hydrolysis, whereas when the halides are present on non-terminal carbon, ketones are produced.
Note:
1,1-dichloroethane is also known as Ethylidene halide, in which both the halide groups are present on the same carbon atom.
Also, the chloride ion is a better leaving group because it is a conjugate base of a strong acid, HCl, which dissociates to give the ion which is very stable.
