Question
Question: \({{(C{{H}_{3}})}_{3}}CBr+KOH\underset{heat}{\overset{ethanol}{\mathop \to }}\,?\)...
(CH3)3CBr+KOHheat→ethanol?
Solution
A type of reaction mainly used to transform saturated organic compounds to unsaturated organic compounds is known as elimination reactions. This method is very important for the preparation of alkenes. Haloalkanes also involved elimination reactions. There are two possible mechanisms available for these elimination reactions – E1 and E2 mechanisms.
Complete step by step solution:
-In elimination reactions of haloalkanes with a β -hydrogen atom on heating with an alcoholic solution of potassium hydroxide leads to loss of a hydrogen atom from the α -carbon atom and a hydrogen tom from β-carbon to form an alkene molecule.
-Given haloalkane is tertiary butyl bromide which belongs to tertiary carbon molecules. -Hence, this organic compound undergoes an E1 mechanism type of elimination reaction.
(CH3)3CBr+KOHheat→ethanol(CH3)2C=CH2+HBr
-In the above reaction, when tertiary butyl bromide undergoes heating alcoholic potassium hydroxide forms 2-methyl prop-1-ene.
-E1 Reaction mechanism of the given reaction: E1 reaction is a unimolecular reaction and the rate-determining step involves the formation of a carbocation intermediate. The stability and reactivity of the carbocation intermediate determine the reactivity of the E1 reaction. The order of reactivity of carbocation intermediate for E1 reaction is 30>20>1o
-Step-1: formation of tertiary carbocation as intermediate. This is a rate-determining step which is a slow reaction.
-Step-2: formation of an alkene by the reaction of tertiary carbocation with alcoholic KOH
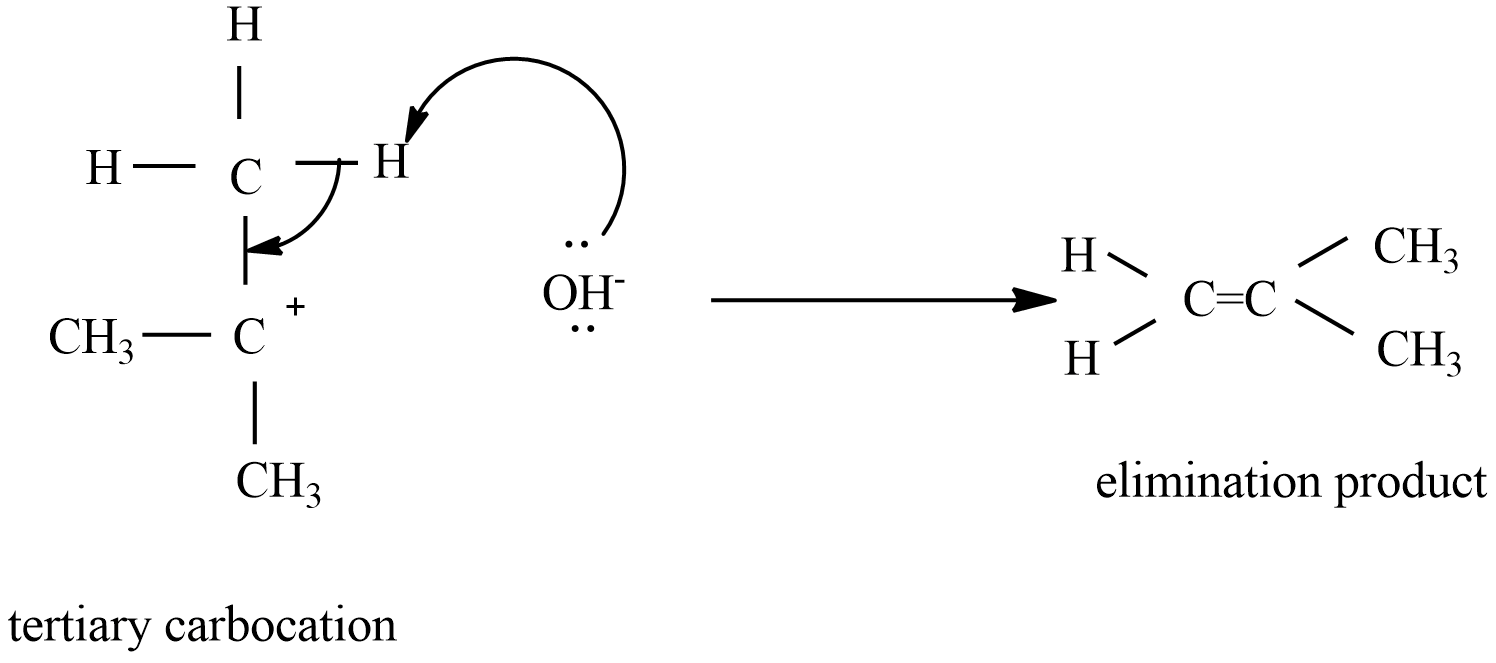
Here the elimination product is 2-methyl prop-1-ene.
Note: The mechanism of elimination reactions mainly consists of three fundamental events: proton removal, C=C bond formed, and there is a breakage in the bond of the leaving group. Depending on the group of atoms or groups leaving from the molecule elimination reactions are distinguished as dehydration and dehalogenation.
