Question
Question: \[C{{H}_{2}}C{{l}_{2}}\] Calculate sigma bonds in the above molecule....
CH2Cl2
Calculate sigma bonds in the above molecule.
Solution
The name of the given compound CH2Cl2 is Methylene chloride and also known as Dichloromethane (DCM). DCM is an organic compound. DCM is a volatile and colourless liquid with a sweet smell. DCM is a saturated compound. The carbon atom forms 2 single bonds with 2 hydrogen atoms and 2 single bonds with 2 chlorine atoms.
Complete step by step answer:
The structure of Dichloromethane is tetrahedral.
t is a derivative of carbon tetrachloride (CCl4).
The hybridization of carbon on DCM is sp3.
The structure of the DCM is as follows.
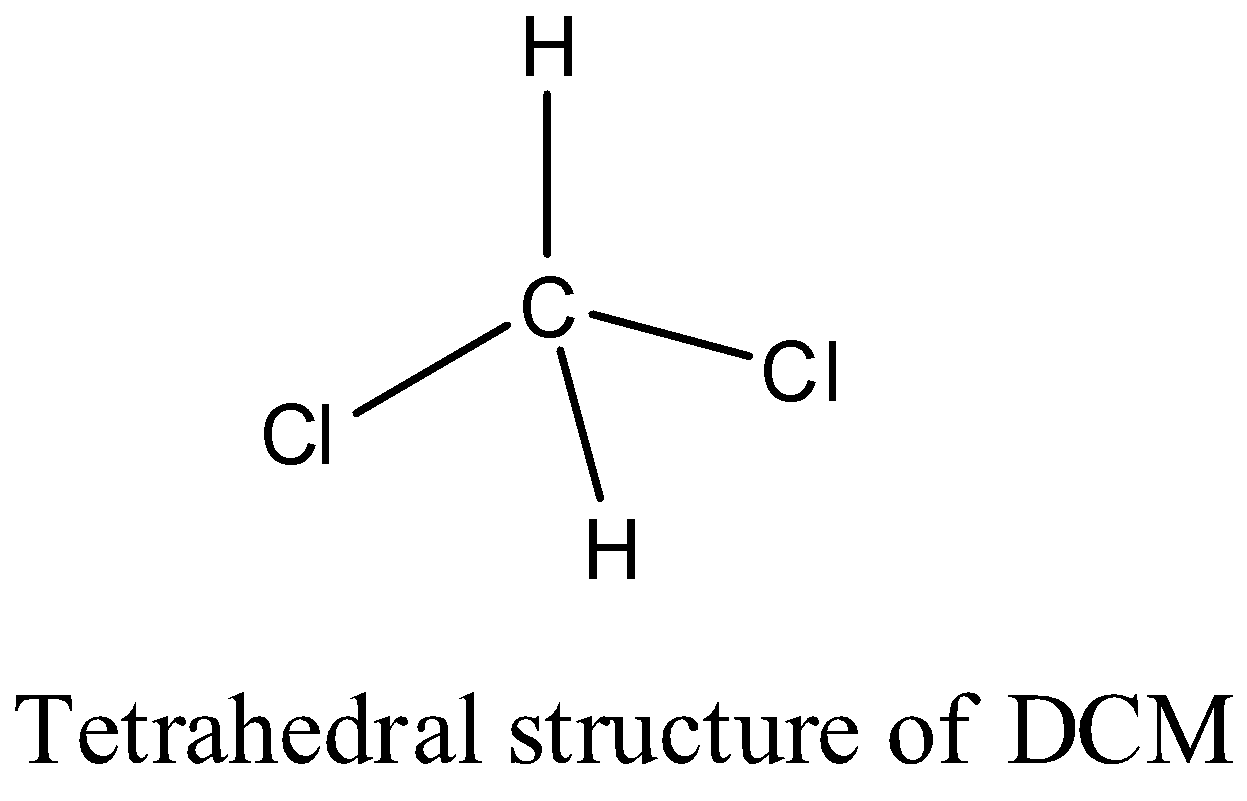
There are two hydrogens and two chlorine atoms in DCM.
We can see that there are four sigma bonds in DCM and there are no pi bonds in the structure of DCM.
The carbon in Dichloromethane is going to form two sigma bonds with two chlorine atoms and two sigma bonds with two hydrogen atoms.
Therefore, the number of sigma bonds in DCM is 4.
Additional information:
DCM compound is highly volatile in nature, so it can cause severe inhalation hazards.
Extended exposure to DCM can cause faintness, weakness, and headache.
DCM undergoes metabolism in the human body and forms carbon monoxide. The formed carbon monoxide in the body leads to carbon monoxide poisoning.
Note:
The compound DCM naturally derived from the volcanoes and from wetlands. It is used as a solvent in the synthesis of many molecules in industries.
DCM is used as a paint remover.
It is also used as a degreasing agent.
DCM is used in the manufacture of aerosol formulations.
DCM is a polar compound but not soluble in water.
