Question
Question: Treatment of compound Ph-O-C-Ph with NaOH solution yield :-...
Treatment of compound Ph-O-C-Ph with NaOH solution yield :-
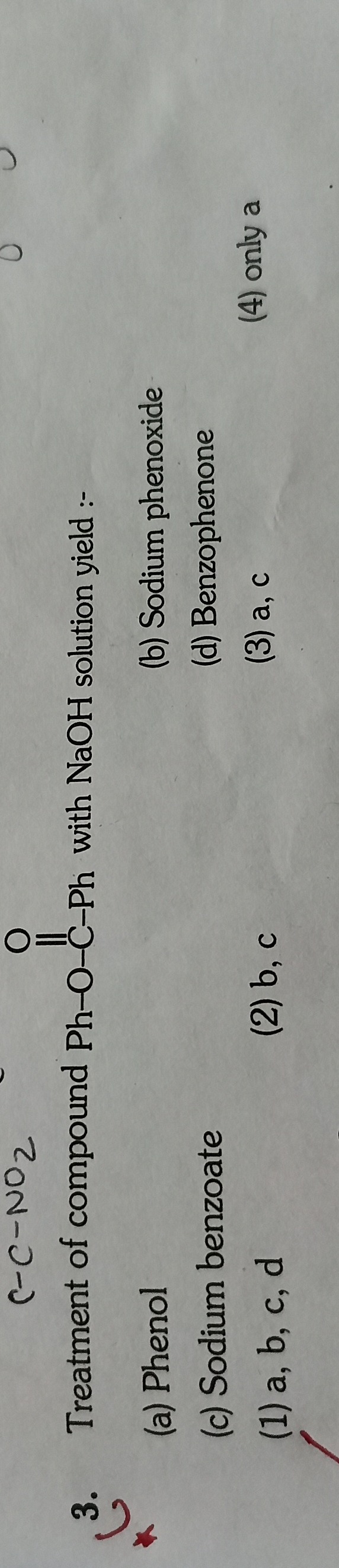
A
Phenol
B
Sodium phenoxide
C
Sodium benzoate
D
Benzophenone
Answer
Sodium phenoxide, Sodium benzoate
Explanation
Solution
The compound Ph-O-C-Ph is phenyl benzoate, an ester. When treated with NaOH solution, it undergoes saponification (basic hydrolysis). The ester linkage breaks to form the phenoxide ion (Ph-O⁻) and the benzoate ion (Ph-COO⁻). In the presence of NaOH, these ions exist as their sodium salts: sodium phenoxide (Ph-ONa) and sodium benzoate (Ph-COONa). Phenol is acidic and will be deprotonated by NaOH to form sodium phenoxide, so phenol itself is not the final product. Benzophenone is not formed from this reaction.
