Question
Question: \({{C}_{6}}{{H}_{5}}S{{O}_{3}}H\xrightarrow{{{H}_{2}}O}A\xrightarrow{{{H}_{2}}/Ni}B\), then A and B ...
C6H5SO3HH2OAH2/NiB, then A and B respectively:
(A) C6H6, Cyclohexane
(B) C6H6, Cyclohexene
(C) C6H6, Cyclohexadiene
(D) C6H5OH, Cyclohexane
Solution
The reactant undergoing the change is benzene sulphonic acid.
- The mixture of hydrogen along with Ni is the reagent used for hydrogenation.
Complete step by step answer:
So in the question a chemical reaction is given and we have to predict the products formed in each step of the reaction. From the time we are studying organic compounds we have gone through many reagents and many conversion mechanisms, let's brush up a few reactions from our memory.
- So first identify the compound which is undergoing the change. The reactant here is the benzene sulphonic acid and it is getting attacked by water.
Benzene sulphonic acid is a very strong acid which readily gets attacked by water and undergoes the desulfonylation group ie the sulphonyl group-−SO3H is getting removed from the molecule.
- So to undergo desulfonylation the required condition is that the reaction must be done in the presence of high concentration of water and heat must be supplied for the reaction with very minimal concentration of sulphuric or say any general acids.
So let’s trace the mechanism of the reaction:
- In the desulfonylation reaction first the water which is a weak base attacks the –OH group of the sulfonyl groups and abstracts the proton and forms the conjugate base of benzene sulphonic acid. And then the double bond in the benzene acts as the nucleophile and attacks the H3O+ and in the next step the heat is the very required element since at this step the sulphonyl group is removed as SO3.
The mechanism of the reaction is as follows:
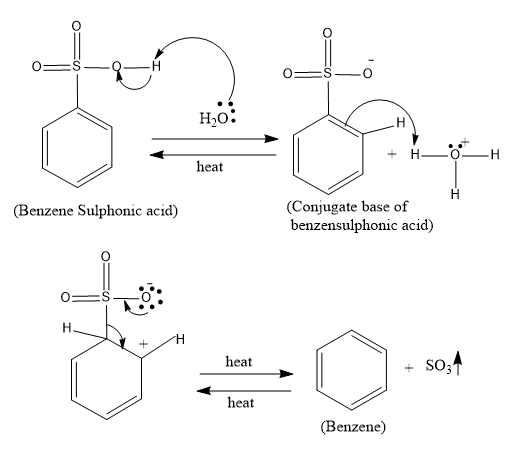
So the overall reaction is as follows:
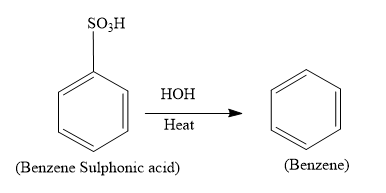
Hence the product (A) formed is benzene which has the formulae, C6H6
- Now let’s move to the next section, here the reagent used is hydrogen and Ni.As we are very familiar that this mixture is used for the hydrogenation of a molecule ie to add on hydrogen to the multiple bonds or we can say that the multiple bonds are getting reduced.
So just add the hydrogen atom in between the double bonds.
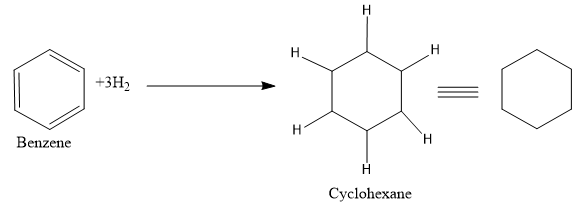
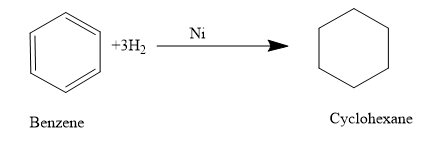
So the product (B) formed is cyclohexane The correct answer is option “A” .
Additional Information :
Note: The reaction is the reverse reaction of the sulfonation reaction of benzene molecules. In the sulphonation of benzene molecules we treat the molecule with sulphuric acid to yield benzene sulphonic acid as the final product.
The other hydrogenation reagent used is Pd/C with H2
