Question
Question: \( {C_6}{H_{12}}(A) \) on treatment with \( HCl \) produces a compound \( Y \) , which is optically ...
C6H12(A) on treatment with HCl produces a compound Y , which is optically inactive. What is the structure of A ?
Solution
An optically inactive compound, is one which does not show optical rotation. Optical activity of an organic compound refers to the property of an organic compound by virtue of which, when it is passed through their solutions, the plane polarized light (created by transmitting ordinary light via Nicol prism) rotates and the compounds are known as optically active compounds. Optically active compounds must possess a chiral centre.
Complete Step-by-Step solution:
Chirality is defined as a 'chiral or stereo centre' object that is asymmetric and cannot be superimposed over its mirror image. This characteristic is known as chirality. This behaviour of chirality is also observed in several organic compounds. Chirality is due to molecules' three-dimensional or spatial arrangements.
If it is superimposable in its mirror image, a molecule is achiral. Many achiral molecules do have a symmetry plane or a symmetry centre. Achiral molecules containing a stereo enter are referred to as meso.
Now, let us write the reaction of the treatment of C6H12 with HCl
C6H12+HCl→C6H13−Cl
C6H12 on treatment with HCl produces an achiral carbon compound as shown in the figure below
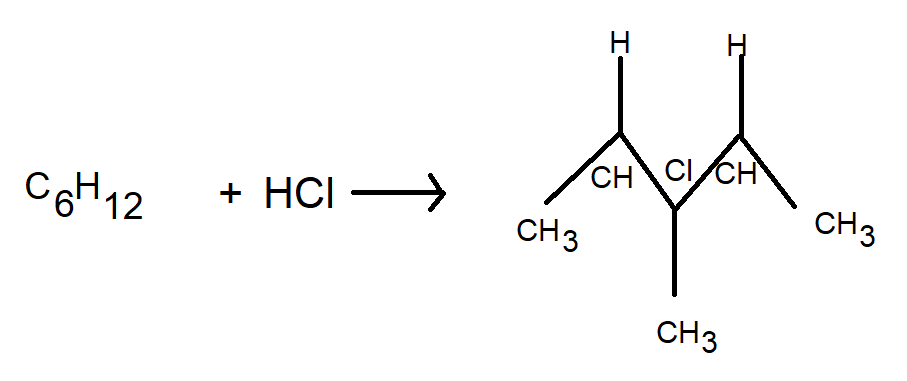
Here, C6H13−Cl is an optically inactive compound. This information was provided to us in the question. It is an achiral compound because we can observe in the figure that there are similar groups on both sides of the chlorine atom. Hence, the name achiral compound. It is said that a compound incapable of optical rotation is optically inactive. All pure achiral compounds are inactive optically.
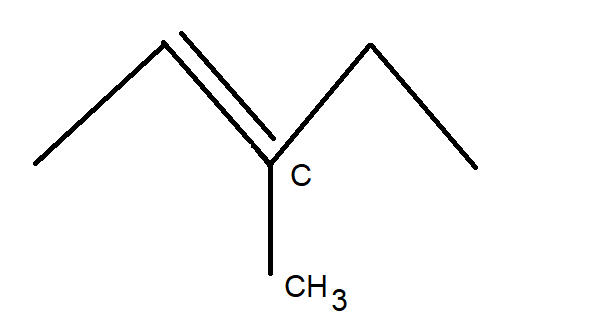
Now, using basic concepts of carbon compounds, let us try to draw the structure of the initial compound C6H12 . The structure of C6H12 can be drawn as shown in the figure below
Note:
If the light moves to the right, the optical activity of the dextrorotatory type is shown, and if it moves to the left, the optical activity of the laevorotatory type is shown. And as the band of light passes through Nicol's lens, it is unaltered. It's claimed to be inert optically. Optical activity, a substance's ability to rotate the polarization plane of a beam of light that passes through it. Since the specific rotation depends on the light's temperature and wavelength, it is also necessary to specify these quantities.
