Question
Question: \({{C}_ {4}} {{H}_ {8}} {{O}_ {2}} \) represents: (A) an acid only (B) an ester only (C) a ket...
C4H8O2 represents:
(A) an acid only
(B) an ester only
(C) a ketone only
(D) an acid and an ester also
Solution
Functional groups are specific substituents or moieties within molecules that may be responsible for the characteristic chemical reactions of those molecules. The same functional group will undergo the same or similar chemical reaction regardless of the size of the molecule it is a part of.
Complete step by step solution:
We have been provided with C4H8O2,
The IUPAC name of the given compound is ethyl acetate,
Ethyl acetate is the organic compound with the formula C4H8O2. This colourless liquid has a characteristic sweet smell and is used in glues, nail polish removers, and in the decaffeination process of tea and coffee.
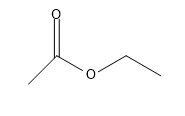
We need to tell the functional group attached to it:
So, we know that: A group of atoms whose bonding is the same from molecule to molecule. A functional group has similar behaviour regardless of the molecule that contains it, so molecules with identical functional groups tend to have similar chemical and physical properties.
So, in ethyl acetate -COOH functional group is attached,
Carboxylic acids are a homologous series in which the compounds contain a functional group called the carboxyl group (-COOH). The general molecular formula for carboxylic acids is CnH2n+1COOH. Carboxylic acids contain at least one carboxyl group.
So, from this we can say that C4H8O2 represents acid only.
Therefore, we can conclude that option (A) is correct.
Note: Ethyl acetate is highly flammable, as well as toxic when ingested or inhaled, and this chemical can be seriously damaging to internal organs in the case of repeated or prolonged exposure. Ethyl acetate can also cause irritation when it comes into contact with the eyes or skin.
