Question
Question: \[C_3H_9N\] cannot represent: A.\[{1^0}\] amine B.\[{2^0}\] amine C.\[{3^0}\] amine D.quater...
C3H9N cannot represent:
A.10 amine
B.20 amine
C.30 amine
D.quaternary salt
Solution
We have the compound with the molecular formula C3H9N. We can use the basics of isomers here. Make the isomers of a compound with the molecular formula C3H9N. Then classify them as primary, secondary, and tertiary amines.
Complete step by step answer:
We will try to draw some isomers of a compound with the molecular formula C3H9N. We will consider and understand all the options one by one. So let’s start.
We can write the structure of the compound with molecular formula C3H9N as,
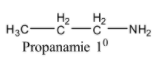
The above structure is a primary 10 amine. As the nitrogen atom is surrounded by a single carbon or chain. So we can represent C3H9N as 10 amine.
Now consider another structure with the molecular formula C3H9N which is represented as,
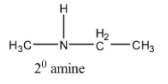
The above structure is 20 amine. Here the nitrogen atom is surrounded by two carbon atoms. So we can represent C3H9N as 20 amine.
Now we will consider another structural isomer of molecular formula C3H9N which is represented as,
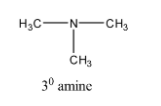
The above structure is a 30 amine. In the structure, we can observe that the nitrogen atom is surrounded by three carbon atoms therefore it is termed as 30 amine. So we can represent C3H9N as 30 amine.
Now the last option is quaternary salt. This salt is represented as R4N+Z− where R4 represents the alkyl group with 4 carbon atoms. But in the compound with the molecular formula, C3H9N we have 3 carbon atoms. So we cannot represent C3H9N it as quaternary salt.
Therefore, the correct option is (D).
Note:
R4N+Z− is the quaternary ammonium halide. It is formed when ammonia accepts one proton to form an ammonium ion and the tertiary amine accepts an alkyl group to form a quaternary ammonium ion.
Primary, secondary and tertiary amines are represented as 10,20,30 amine respectively.
