Question
Question: C-3. Effective overlapping will be shows by:...
C-3. Effective overlapping will be shows by:
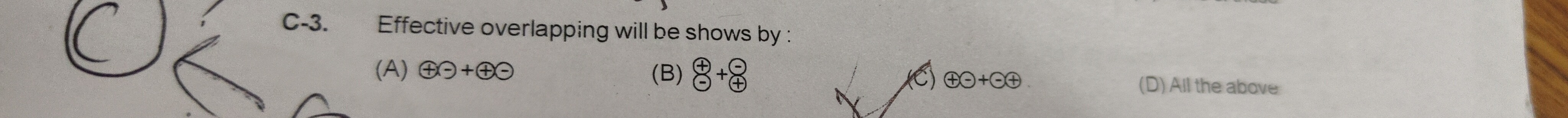
⊕⊖+⊕⊖
⊖⊕ + ⊕⊖
⊕⊖+⊖⊕
All the above
A
Solution
Effective overlapping occurs when atomic orbital lobes of the same phase overlap, leading to constructive interference and bond formation.
-
(A) ⊕⊖+⊕⊖: This represents two p-orbitals where same-phase lobes can overlap (e.g., ⊕ with ⊕, and ⊖ with ⊖) for both sigma (head-on) and pi (sideways) bond formation. This is effective overlap.
-
(B) ⊖⊕ + ⊕⊖: This represents sideways overlap where the upper ⊕ lobe of one orbital overlaps with the upper ⊖ lobe of the other, and similarly for the lower lobes. This is opposite-phase overlap, leading to destructive interference (antibonding orbital). This is not effective overlap.
-
(C) ⊕⊖+⊖⊕: This represents two p-orbitals where the phases are inverted in the second orbital relative to the first. Any head-on or sideways overlap will involve lobes of opposite phases (⊕ with ⊖), leading to destructive interference (antibonding orbital). This is not effective overlap.
Thus, only (A) shows effective overlapping.
