Question
Question: \(But - 2 - ene\) on reaction with alkaline \(KMn{O_4}\) at elevated temperature followed by acidi...
But−2−ene on reaction with alkaline KMnO4 at elevated temperature followed
by acidification will give:
(A) One molecule of CH3CHO and one molecule of CH3COOH
(B)
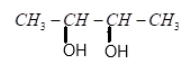
(C) 2 molecules of CH3COOH
(D) 2molecules of CH3CHO
Solution
As we know that alkene can react with cold, acidic, and alkaline potassium permanganate and form different products so alkaline KMnO4 being an oxidising agent when added to an alkene it results in the ketonic or acidic compounds formation.
Complete Step by step answer: As we know that the carbon-carbon double bond in alkene reacts with the alkaline potassium permanganate followed by acidification and results into a ketone or acidic compound formation. Alkene on reaction with cold, dilute or aqueous solution of potassium permanganate also known as Beyer’s reagent, produces vicinal glycols.
So, But−2−ene on reaction with potassium permanganate oxidises the compound and results in the formation of two molecules of Ethanoic acid.
CH3−CH=CH−CH3KMnO42CH3COOH
We can show this reaction as:
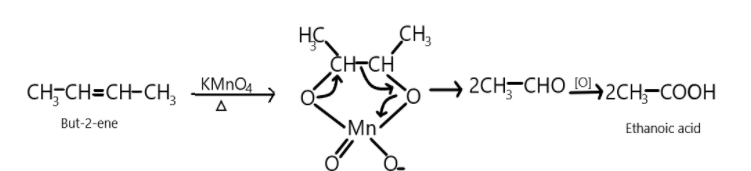
So first alkene reacts with potassium permanganate and breakdown or double bonds take place resulting into the formation of two aldehydic compounds which on further oxidation produces two molecules of ethanoic acid which is the main product in the following reaction.
Therefore the correct answer is (C).
Note: potassium permanganate is a crystalline solid which is purple-black in colour and discolouration of potassium permanganate solution is used as a test for unsaturation. Alkaline potassium permanganate can be used to identify whether the organic compound is an alkene or an alkane.
