Question
Question: Buckminsterfullerene is an allotropic form of: (A) phosphorus (B) Sulphur (C) carbon (D) tin...
Buckminsterfullerene is an allotropic form of:
(A) phosphorus
(B) Sulphur
(C) carbon
(D) tin
Solution
Some substances exist in more than one physical form. They differ in the arrangement of atoms in crystalline solid. Such substances are called allotropes.
They differ in physical properties such as color, hardness but usually alike in most chemical properties.
Complete step by step answer:
Element carbon exists in three different allotropic forms.
They are Diamond, Graphite and Buckminsterfullerene.
Buckminsterfullerene is an allotropic form of carbon.
So, the correct answer is “Option C”.
Additional Information:
This isomer is also known as Buckminsterfullerene has a molecular formula as C60 and has a shape of soccer ball i.e., hollow sphere.
There are sixty equidistant spaces on the sphere that are occupied by C-atoms.
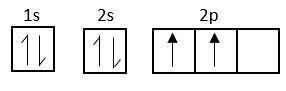
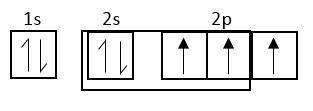
Each c-atom of fullerene is sp2 hybridized
Electronic configuration of carbon is, sp22s22px12py12pz1
C-atom at ground state
At excited state
Hybridized state
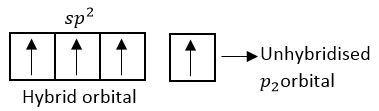
2pz orbitals form delocalized molecular orbital.
They spread over the complete structure of fullerene.
Carbon atoms are arranged in hexagons and pentagons and form hollow spheres.
Fullerene is observed in root.
When a high power laser was focused on carbon fullerene is formed.
Note:
Compounds of fullerene K35C60 act as superconductors of electricity.
It reacts with transition metal to form a catalyst Nanotubes are made from fullerene and graphite.
They are used in electric conductors, molecular sensors and semiconductors.
(1) Allotropes of phosphorus are white phosphorus, Red phosphorus, black phosphorus.
(2) Allotropes of Sulphur are Rhombic Sulphur, monoclinic Sulphur, α-sulphur, β-sulphur plastic sulphur etc.
(3) Tin exists in two forms i.e., white or beta and gray or alpha tin.
