Question
Question: Bring about the following conversion: 1-pentene to 1-pentyne....
Bring about the following conversion:
1-pentene to 1-pentyne.
Solution
In the above question, it is asked to convert 1-pentene to 1-pentyne. This can be done by adding bromine to 1-pentene followed by dehydrobromination in the presence of soda amine. This dehydrobromination is an example of double elimination reaction.
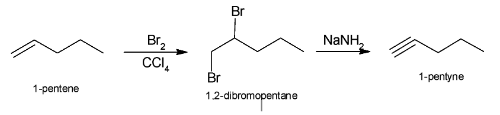
Complete step-by-step answer: In the above question since we have to convert 1-pentane to1-pentyne, so we have to first convert it to 1,2-dibromo pentane such that after adding NaNH2, double elimination takes place resulting in formation of 1-pentyne.
So, the first step include conversion of 1-pentene to 1,2-dibromopentane with the help of Br2 and CCl4. The above reaction can be illustrated as:
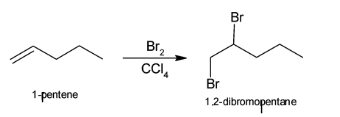
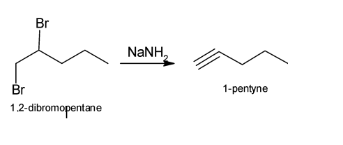
NaNH2 helps in the formation of alkynes from halogens. Treatment of either geminal dihalides (i.e., two halogens on one carbon) or vicinal dihalides (halogens on adjacent carbons) with NaNH2 results in the formation of alkynes. Hence, when vicinal dihalides or 1,2-dibromopentane reacts with
NaNH2, 1-pentyne is formed.
The above reaction can be illustrated as:
The complete reaction of conversion of 1-pentene to 1-pentyne can be illustrated as:
Note: Aromatic compounds require lewis acid likeAlCl3 for bromination. But the reaction that will take place will be substitution instead of addition.
Alkanes do not react with Br2.
NaNH2 is a strong base. It can be an excellent nucleophile. It is used for deprotonation of weak acids and also for elimination reactions.
As a strong base, NaNH2 will deprotonate alkynes, alcohols, and a host of other functional groups with acidic protons such as esters and ketones.
