Question
Question: Which of the following reaction is wrong?...
Which of the following reaction is wrong?
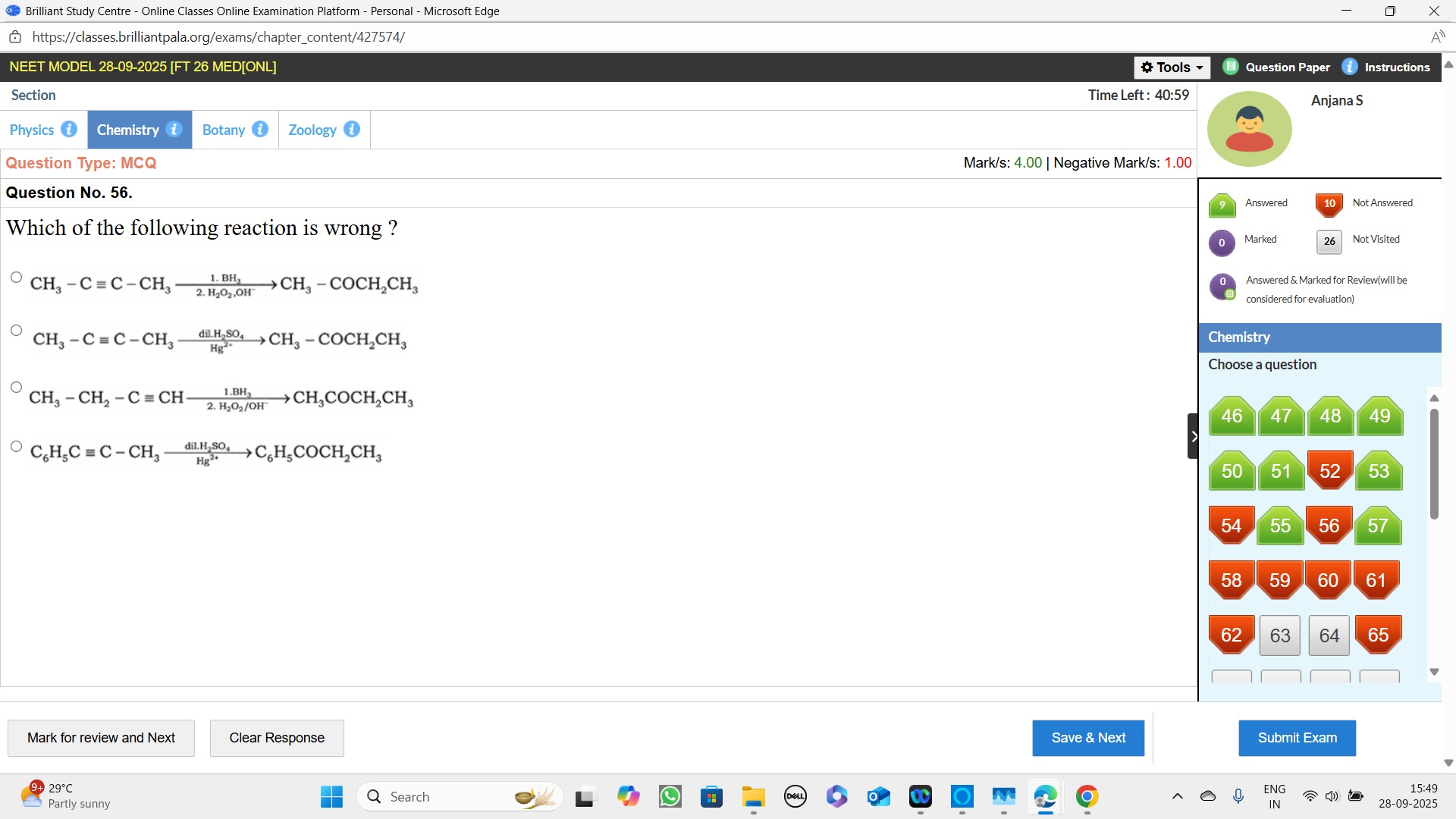
CH_3 - C \equiv C - CH_3 \xrightarrow[2.H_2O_2,OH^-]{1. BH_3} CH_3 - COCH_2CH_3
CH_3 - C \equiv C - CH_3 \xrightarrow[Hg^{2+}]{dil. H_2SO_4} CH_3 - COCH_2CH_3
CH_3 - CH_2 - C \equiv CH \xrightarrow[2.H_2O_2/OH^-]{1. BH_3} CH_3COCH_2CH_3
C_6H_5C \equiv C - CH_3 \xrightarrow[Hg^{2+}]{dil. H_2SO_4} C_6H_5COCH_2CH_3
CH_3 - CH_2 - C \equiv CH \xrightarrow[2.H_2O_2/OH^-]{1. BH_3} CH_3COCH_2CH_3
Solution
The question asks to identify the incorrect reaction. Let's analyze each option:
-
Option 1: CH3−C≡C−CH31.BH32.H2O2,OH−CH3−COCH2CH3 This is the hydroboration-oxidation of but-2-yne (an internal alkyne). Hydroboration-oxidation of internal alkynes yields ketones. The product, butan-2-one (CH3COCH2CH3), is correct.
-
Option 2: CH3−C≡C−CH3dil.H2SO4Hg2+CH3−COCH2CH3 This is the acid-catalyzed hydration of but-2-yne (an internal alkyne) using dilute H2SO4 and Hg2+. This reaction yields a ketone. The product, butan-2-one (CH3COCH2CH3), is correct.
-
Option 3: CH3−CH2−C≡CH1.BH32.H2O2/OH−CH3COCH2CH3 This is the hydroboration-oxidation of but-1-yne (a terminal alkyne). Hydroboration-oxidation of terminal alkynes leads to aldehydes via anti-Markovnikov addition. The expected product is butanal (CH3CH2CH2CHO). The product shown, butan-2-one (CH3COCH2CH3), is incorrect.
-
Option 4: C6H5C≡C−CH3dil.H2SO4Hg2+C6H5COCH2CH3 This is the acid-catalyzed hydration of 1-phenylprop-1-yne (an unsymmetrical internal alkyne). The reaction follows Markovnikov's rule, and the enol formed tautomerizes to a ketone. The phenyl group stabilizes the carbocation intermediate, leading to the hydroxyl group adding to the carbon adjacent to the phenyl group. The expected product is 1-phenylpropan-1-one (C6H5COCH2CH3), which is correct.
Therefore, the incorrect reaction is in Option 3.
