Question
Chemistry Question on States of matter
Boyle?? temperature or Boyle point is the temperature at which a real gas starts behaving like an ideal gas over a particular range of pressure. A graph is plotted between compressibility factor Z and pressure P. 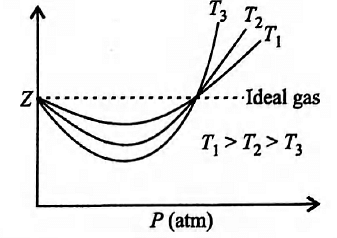 What is the deviation of real gas from ideal behaviour in terms of compressibility factor, Z?
What is the deviation of real gas from ideal behaviour in terms of compressibility factor, Z?
As the temperature increases, Z approaches a value close to one and gas starts behaving ideally.
Z continuously decreases with increase in pressure.
Z continuously increases with increase in pressure.
At high pressure, every gas has value Z=1.
As the temperature increases, Z approaches a value close to one and gas starts behaving ideally.
Solution
Compressibility factor Z is a correction factor which gives the deviation of a real gas from an ideal gas. It is the ratio of the molar volume of a gas to the molar volume of an ideal gas, at same T and P. Here as the temperature increases, Z starts approaching towards one and then the gas starts behaving ideally.
