Question
Question: $\boxed{ }$C$\equiv$C-H$\xrightarrow[\text{dil. H}_2\text{SO}_4]{\text{HgSO}_4}$[A]$\xrightarrow[\te...
C≡C-HHgSO4dil. H2SO4[A]Zn-HgConc. HCl[B]Cl2AlCl3[C]
Compound (C) can be:
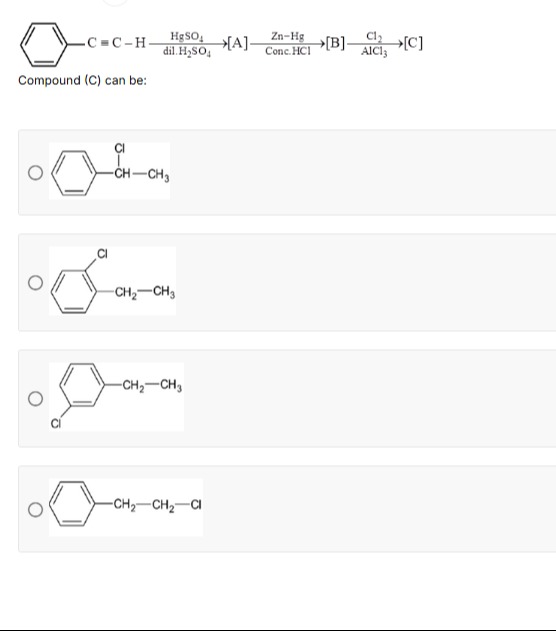
A benzene ring is connected to a carbon atom, which is connected to a chlorine atom and a methyl group. The carbon atom is bonded to the benzene ring and the chlorine atom with single bonds. The methyl group is bonded to the carbon atom with a single bond.
A benzene ring is connected to a methylene group, which is connected to a methyl group. The methylene group is bonded to the benzene ring with a single bond. The methyl group is bonded to the methylene group with a single bond. A chlorine atom is bonded to the benzene ring at the ortho position relative to the methylene group.
A benzene ring is connected to a methylene group, which is connected to a methyl group. The methylene group is bonded to the benzene ring with a single bond. The methyl group is bonded to the methylene group with a single bond. A chlorine atom is bonded to the benzene ring at the meta position relative to the methylene group.
A benzene ring is connected to an ethyl group, which is connected to a chlorine atom. The ethyl group is bonded to the benzene ring with a single bond. The chlorine atom is bonded to the ethyl group with a single bond.
Option 2
Solution
-
Hydration:
Starting from phenylacetylene,
Ph–C≡C–Hdil. H2SO4,HgSO4 acetophenone:
Ph–CO–CH3 -
Clemmensen reduction:
Acetophenone is reduced by Zn–Hg/conc. HCl to give ethylbenzene:
Ph–CO–CH3Zn–Hg, Conc. HClPh–CH2CH3 -
Friedel–Crafts Chlorination:
Treatment of ethylbenzene with Cl2 in the presence of AlCl3 (an electrophilic aromatic substitution) produces a chlorinated ethylbenzene. Since the ethyl group is an ortho/para director (and meta substitution is ruled out), the chlorine electrophile attacks the aromatic ring at the activated positions. Although para substitution is often predominant due to steric reasons, among the options provided the only aromatic substituted possibilities are shown with chlorine at positions relative to the alkyl (methylene) substituent.
Option 2 shows the benzene ring bearing a –CH₂CH₃ group with chlorine attached to the ring at the ortho position (relative to the –CH₂– group). This is consistent with electrophilic substitution by the Cl⁺ generated by Cl2/AlCl3.
