Question
Question: Both phosphinic acid and phosphonic acids have A. One \(P=O\) bond B. Two \(P-H\) bond C. Two ...
Both phosphinic acid and phosphonic acids have
A. One P=O bond
B. Two P−H bond
C. Two P−OH bond
D. One P−O−P bond
Solution
Phosphinic or phosphonic acids both are phosphorous oxyacid. Phosphinic acid also known by the name hypophosphorous acid is generally colorless low melting point while phosphonic acid can be known as the phosphonates these are generally organophosphorus compounds.
Complete answer: Phosphinic acid is represented by the molecular formula H3PO2 which is used as a reducing agent. It is soluble in water, dioxane and alcohol. It generally represents in the form of HOP(O)H2 which defines its monoprotic character or we can say acidic character. At equilibrium HOP(O)H2 exists with the minor tautomer HP(OH)2. The structure of phosphonic acid can be shown as:
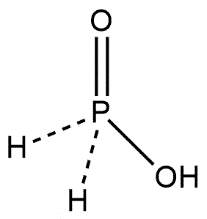
Phosphonic acids are also known by the name phosphonates and these are kept in the category of organophosphorus compounds. Phosphonic acids are represented by the molecular formula containing R−PO3H2 group where R may be an alkyl or aryl group. Phosphonic acid consists of a single pentavalent phosphorus covalently bound via single bonds to single hydrogen and two hydroxyl groups via a double bond to an oxygen. Structure of phosphonic acid can be shown as:
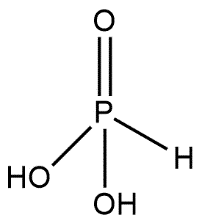
In both of the structures we can consider that one P=O bond is common.
Hence we can say that option A is the correct answer.
Note: The main use of Phosphinic acid is its industrial use for electroless nickel plating but it can be used as a salt. It also reduces chromium(III) oxide to chromium(II) oxide. Phosphonic acid is a conjugate acid of a phosphonate and a tautomer of a phosphorous acid.
