Question
Question: Boric acid is polymeric due to: A) Its acidic nature B) The presence of hydrogen bonds C) Its ...
Boric acid is polymeric due to:
A) Its acidic nature
B) The presence of hydrogen bonds
C) Its monobasic nature
D) Its geometry
Solution
Boric acid also called hydrogen borate, boracic acid, and orthoboric acid. It is a weak, monobasic(one hydrogen as proton donor) Lewis acid of boron. Some reaction shows that it is a tribasic acid in bronsted theory.
Complete answer:
Boron can form many compounds. There are some useful compounds to borons as well. These are borax, orthoboric acid and diborane.
Borax is most important. It is white crystalline solid of formula Na2B4O7.10H2O . Borax dissolves in water and gives alkaline solution. The simplest boron hydride is known as diborane, prepared by treating boron trifluoride with lithium aluminium hydride.The orthoboric acid H3BO3 is also white crystalline solid, with soapy touch. It is sparingly soluble in water but highly soluble in water. It can be prepared by acidifying an aqueous solution of borax
Na2B4O7+2HCl+5H2O→2NaCl+4H3BO3
Structure of boric acid- Orthoboric acid has a layer structure in which planar BO3 units are joined by hydrogen bonds. Structure of boric acid as-
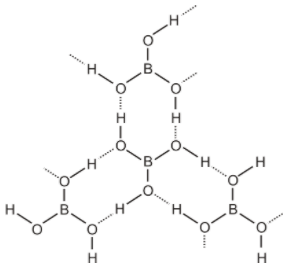
Here the dotted line represents hydrogen bonds.
Boric acid is a weak monobasic acid since it is not able to release hydrogen ion on its own, it receives hydroxyl ion from water, completes its octet and then releases hydrogen ion. It is not a protonic acid, but acts as Lewis acid by accepting an electron pair from hydroxyl ions.
On heating, orthoboric acid above 370K forms metaboric acid, which on further heating gives boric oxide. So the correct option is B. as we can see from the figure that it is polymeric due then hydrogen bonds.
Note: Boric acid is very useful. It can be used as antiseptic for minor burns or cuts. Also it can be used as antibacterial in acne treatment. It is effectively used to counteract the harmful effects of reactive hydrofluoric acid after contact with skin.
