Question
Question: Boric acid has a polymeric layer structure in which planar \(B{{O}_{3}}\) units are joined by: (A)...
Boric acid has a polymeric layer structure in which planar BO3 units are joined by:
(A)- covalent bonds
(B)- two centre-two electron bonds
(C)- coordinate bonds
(D)- hydrogen bonds
Solution
Boric acid (H3BO3) in solid crystalline state has two dimensional layered structure. Hydrogen bond is a weak bond between an electronegative atom like N, O, F and a hydrogen atom bonded to another electronegative atom.
Complete answer:
To understand the structure of boric acid (H3BO3), consider the ground state electronic configuration of boron.
B (in ground state): 1s22s22p1
One electron from 2s moves to 2p-orbital in the excited state and the electronic configuration becomes: 1s22s12p2
One s and two p-orbitals are now available to bond with three oxygen atoms. Therefore, the hybridization of the central atom B issp2.
Now, since only one electron of each oxygen atom is used in the bond formation. The borate ion formed is a trivalent ion,BO3−3. Due to sp2 the geometry of BO3−3 is trigonal planar. In the triangular BO3−3, three oxygen atoms are present at the corners of an equilateral triangle.
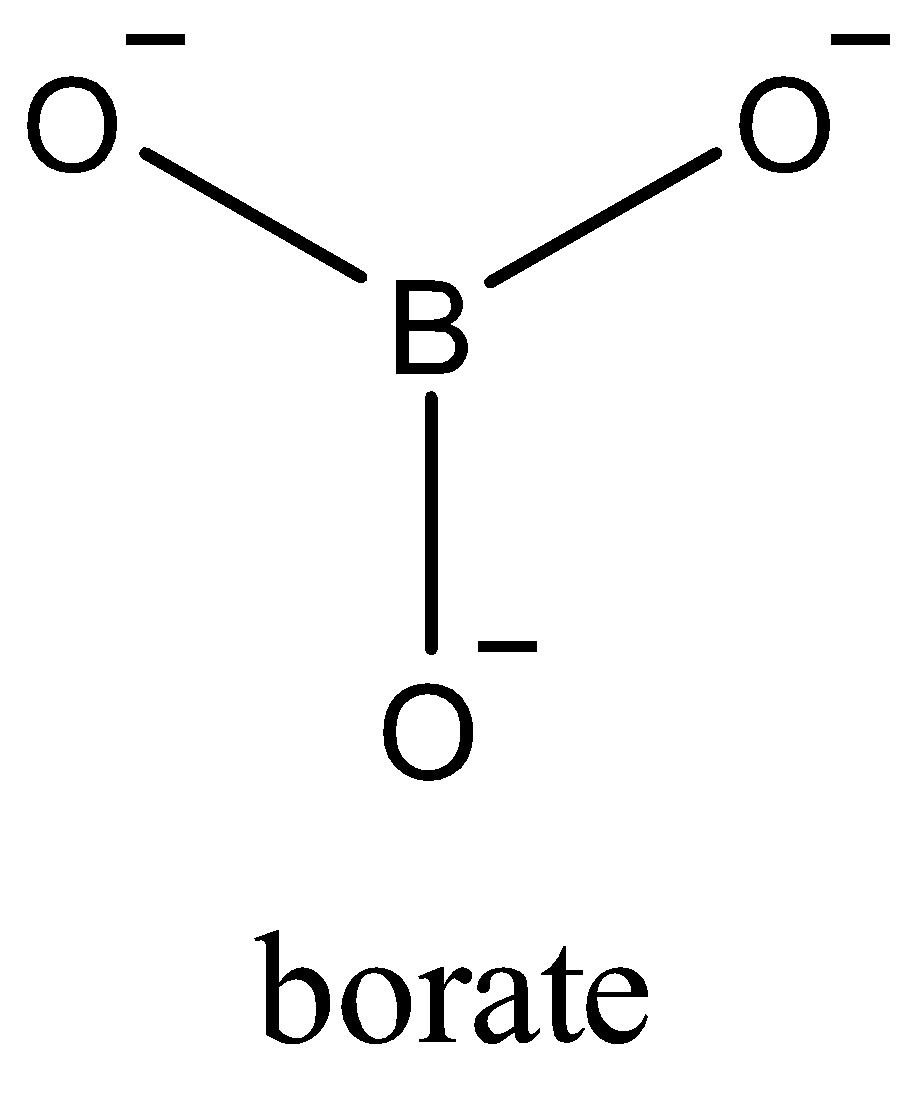
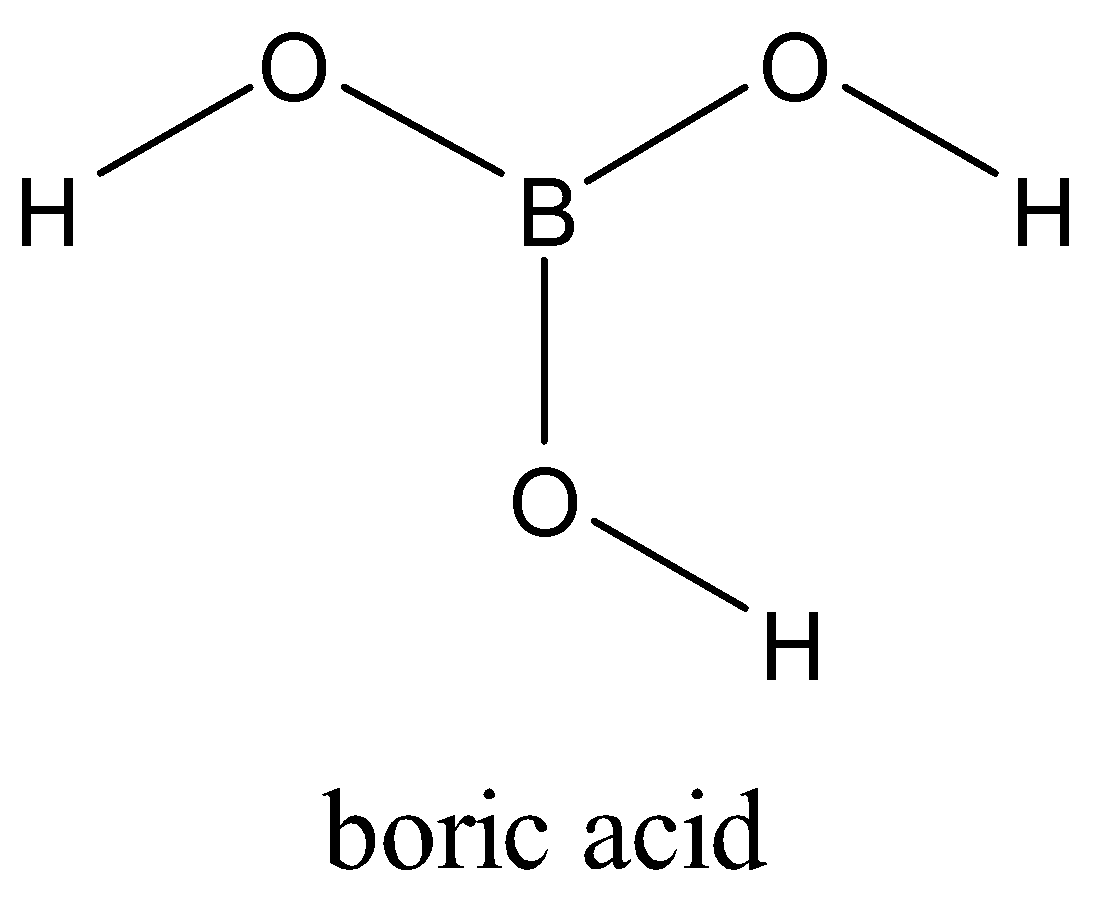
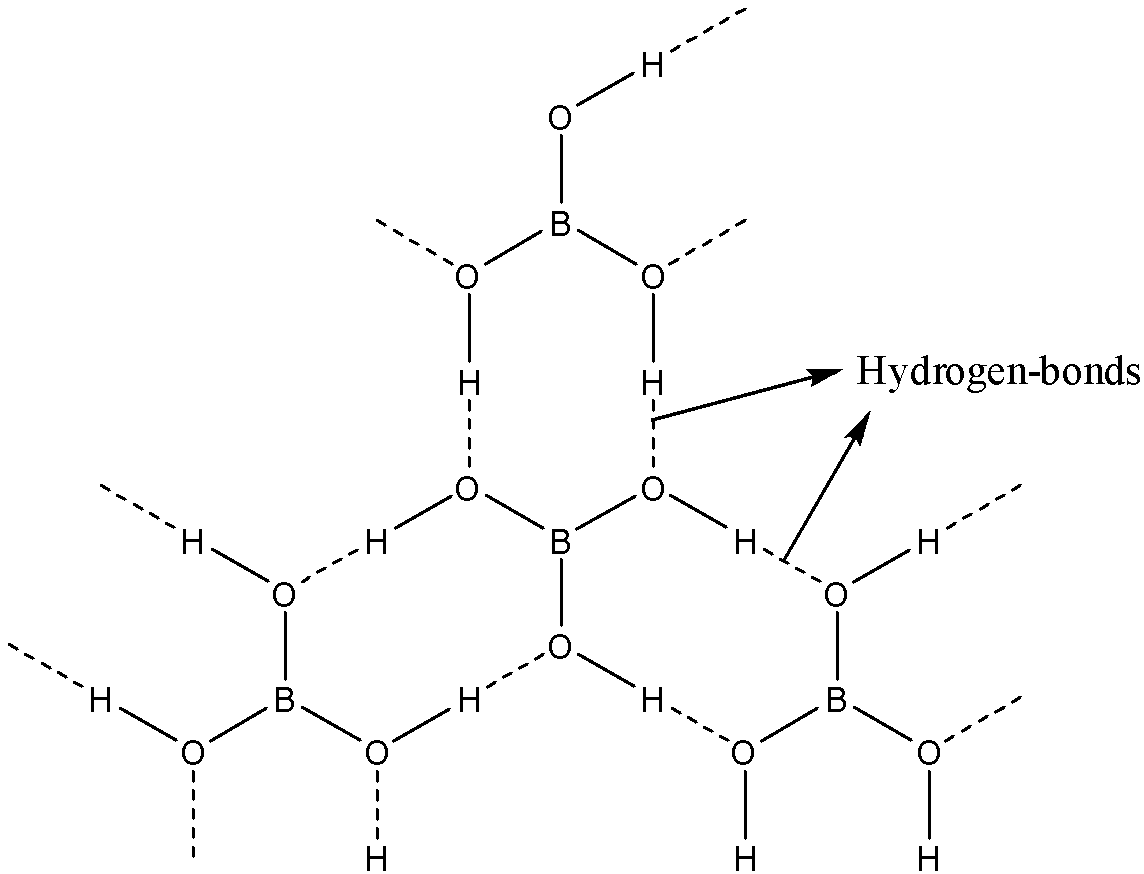
Boron is attached to three oxygen atoms and each oxygen is bonded to one hydrogen. Each such unit is H3BO3. These units are bonded together through hydrogen bonds to give a two-dimensional layered structure of boric acid.
So, the correct answer is “Option D”.
Additional Information: Boric acid or orthoboric acid is a weak monobasic acid. It does not donate protons but accepts a pair of electrons H3BO3 from and thus behaves as a Lewis acid. It has a soapy touch and is moderately soluble in water.
Note: Bonds between boron and oxygen atoms are covalent. One H3BO3 unit is joined to another through hydrogen bonding between oxygen of one H3BO3 to the hydrogen of another H3BO3.
