Question
Question: Borax structure contains? A. Two \(B{{O}_{4}}\) groups and two \(B{{O}_{3}}\) groups B. Four\(B{...
Borax structure contains?
A. Two BO4 groups and two BO3 groups
B. FourBO4 groups only
C. Four BO3 groups only
D. Three BO4 groups and one BO3 group
Solution
Hint : Borax is a compound of boron it has a structure having tetra nuclear units. Borax is a salt that contains water of crystallization. It is an important compound of boron. It gives a famous borax bead test on subjection with heat to form transparent beads like glass. They consist of sodium Meta borate and boric anhydride.
Complete answer:
Borax is an important compound of boron. It has a formula Na2[B4O5(OH)4].8H2O and thus also called as sodium tetraborate. Its structure consist of tetra nuclear units of the formula[B4O5(OH)4]2−
The structure of borax consists of an arrangement with 4 boron atoms such that 2 boron atoms form 3 – coordinate bonds, while the other 2 boron atoms form 4 – coordinate bonds. Thus the structure gives rise to the formation of two groups of boron and oxygen arrangement. The arrangement where 2 boron atoms are in the form of tetrahedral atoms having two BO4groups, and the other arrangement where the other 2 boron atoms have a planar arrangement with 3 oxygen atoms as BO3 groups. The structure of borax is as follows:
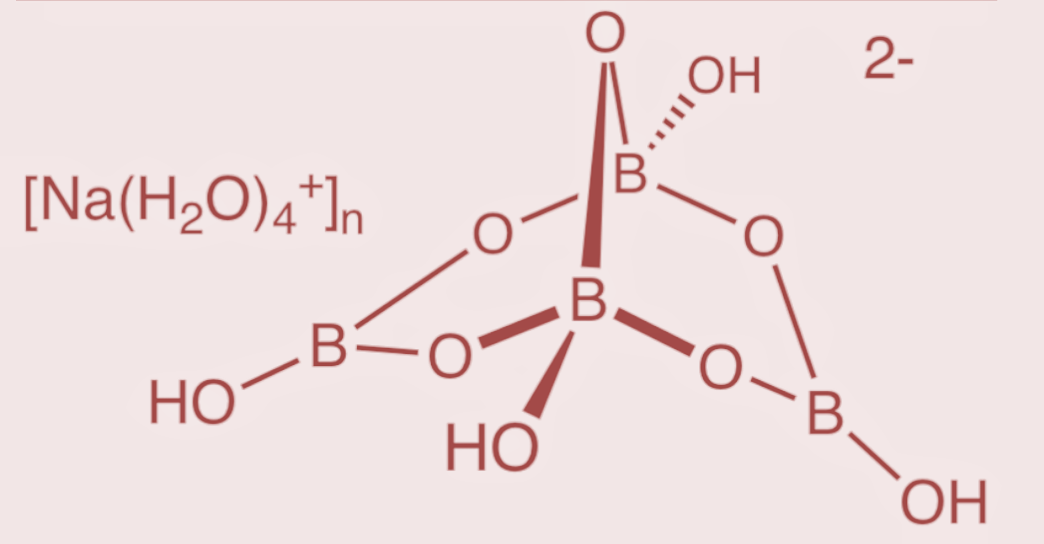
Hence, the borax structure contains two BO4 groups and two BO3groups.
So, option A is correct.
Note:
Borax is one of the most useful compounds of boron. It is used in preparing antiseptic medicinal soaps, for water softening, manufacturing borosilicate glass, soldering etc. It gives a known borax bead test that when subjected to heating it forms glass like beads that are important and useful in qualitative analysis of radicals.
