Question
Question: Bond order of\({{\text{O}}_{\text{2}}}\), \(\text{O}_{\text{2}}^{\text{+}}\), \(O_{2}^{-}\) and \[\t...
Bond order ofO2, O2+, O2− and O22- is in order.
(A) O2- ⟨ O22− ⟨ O2 ⟨ O2+
(B) O22- ⟨ O2− ⟨ O2 ⟨ O2+
(C) O2+ ⟨ O2 ⟨ O2− ⟨ O22−
(D) O2 ⟨ O2+ ⟨ O2− ⟨ O22−
Solution
First draw a molecular orbital diagram (MOT) where the atomic orbitals combine to form molecular orbitals. The total electrons associated with the molecules are filled in the MOT diagram.
To solve this question, we need to write the molecular orbital configuration. To find out the bond order from the molecular orbital configuration is:
Bond order =21[Bonding - antibonding]
Complete step by step answer:
Let’s first draw the MOT of the oxygen molecule. The oxygen molecule O2 contains the 16 electrons. The MOT is as shown below:
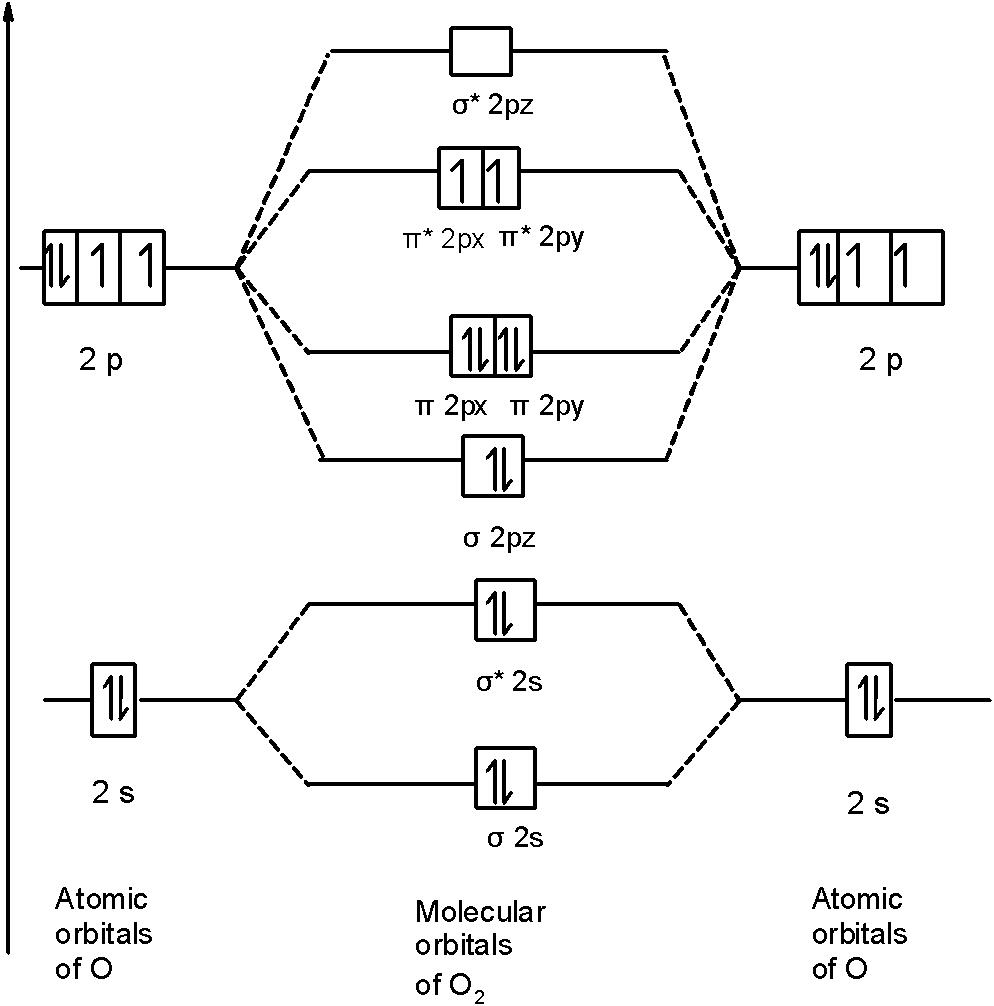
First of all, we can write the molecular orbital configuration of O2 the molecule. In a O2 molecule, there are a total of 16 electrons. The molecular orbital configuration of O2 the molecule is as follows:
$$$$$$\text{ }\!\!\sigma\!\!\text{ 1}{{\text{s}}^{\text{2}}}\text{,}{{\text{ }\!\!\sigma\!\!\text{ }}^{\text{}}}\text{1}{{\text{s}}^{\text{2}}}\text{, }\!\!\sigma\!\!\text{ 2}{{\text{s}}^{\text{2}}}\text{, }{{\text{ }\!\!\sigma\!\!\text{ }}^{\text{}}}\text{2}{{\text{s}}^{\text{2}}}\text{, }\!\!\sigma\!\!\text{ 2}{{\text{p}}^{\text{2}}}{z}\text{, 2p}{\text{x}}^{\text{2}}\text{ }\!\!\pi\!\!\text{ = 2p}{\text{y}}^{\text{2}}\text{ }\!\!\pi\!\!\text{ , 2p}{\text{x}}^{1}{{\text{ }\!\!\pi\!\!\text{ }}^{\text{}}}\text{=2p}_{\text{y}}^{1}{{\text{ }\!\!\pi\!\!\text{ }}^{\text{}}}$$
There are 10 bonding and 6 nonbonding electrons in the orbitals according to the molecular orbital configuration.
Therefore, Bond order =21[Bonding-antibonding]
= 21[10−6]=21(4)=2
Thus, the bond order O2 is 2.
Similarly, in O2+ a molecule, there are 15 electrons. Therefore, the MOT diagram O2+ contains the 15 electrons. We will write down the MOT diagram for the 2p orbital as the bonding between the 1s and 2s is the same as that of the O2 . The MOT diagram is,
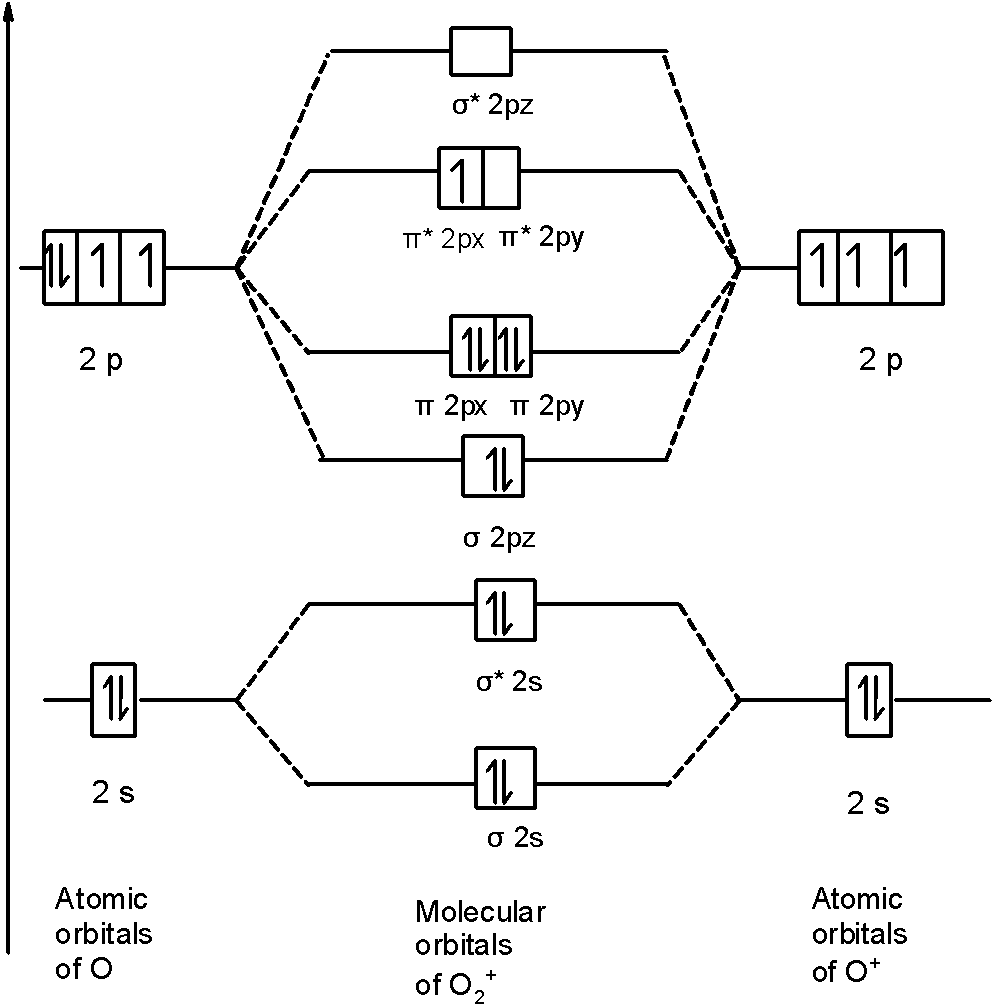
Therefore, the molecular orbital configuration of O2+ is as follows:O2+
!!σ!! 1s2, !!σ!! *1s2, !!σ!! 2s2, !!σ!! *2s2, !!σ!! 2p2z, 2px2 !!π!! = 2py2 !!π!! , 2px2 !!π!! *=2py0 !!π!! *
There are 10 bonding (including molecular orbitals formed by the 1s orbitals) and 5 nonbonding electrons in the orbitals according to the molecular orbital configuration.
Therefore, Bond order =21[Bonding-antibonding]
=21[10−5]=2.5
Therefore, the bond order of O2+ is 2.5.
In O2− a molecule, there are 17 electrons. The MOT diagram holds a total of 17 electrons. The MOT diagram is as shown below,
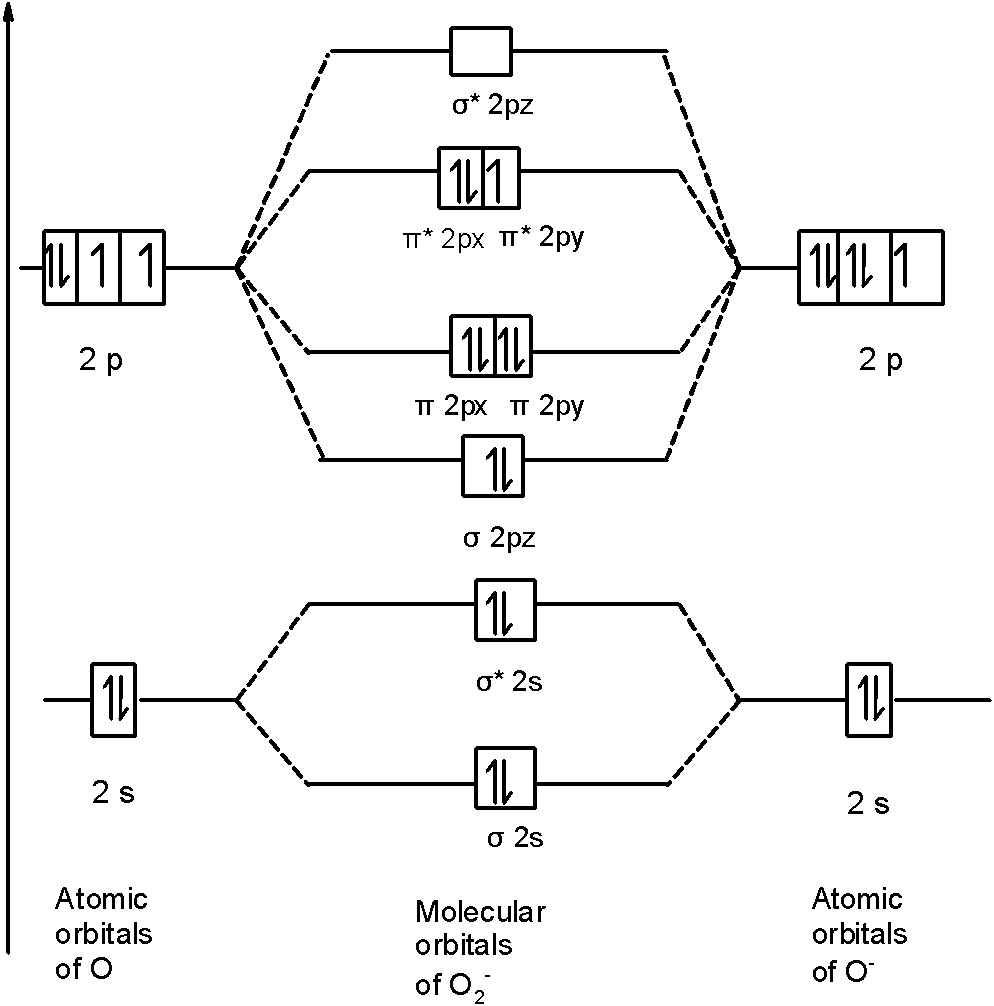
So, the molecular orbital configuration is as follows:
!!σ!! 1s2, !!σ!! *1s2, !!σ!! 2s2, !!σ!! *2s2, !!σ!! 2p2z, 2px2 !!π!! = 2py2 !!π!! , 2px2 !!π!! *=2py1 !!π!! *
There are 10 bonding electrons (including molecular orbitals formed by the 1s orbitals.) and 7 nonbonding electrons.
Therefore, Bond order =21[Bonding-antibonding]
=21[10−7]=1.5
Therefore, the bond order O2− is 1.5.
In O22- a molecule, there are 18 electrons. The MOT diagram is as shown below,
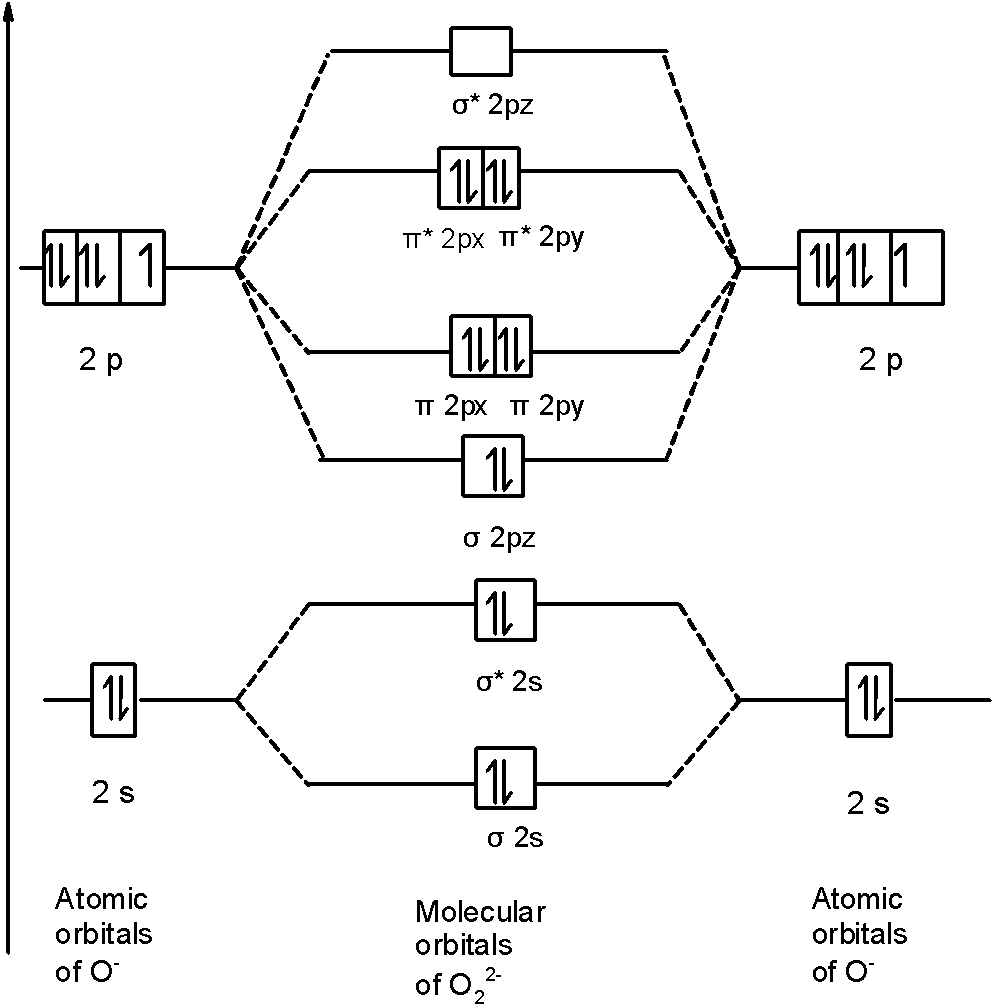
So, the molecular orbital configuration is as follows:
!!σ!! 1s2, !!σ!! *1s2, !!σ!! 2s2, !!σ!! *2s2, !!σ!! 2p2z, 2px2 !!π!! = 2py2 !!π!! , 2px2 !!π!! *=2py2 !!π!! *
There are 10 bonding electrons and 8 nonbonding electrons.
Therefore, bond order =21[Bonding-antibonding]
=21[10−8]=1
Therefore, the bond order O22- is 1.
So, the correct order of bond order is O22- ⟨ O2− ⟨ O2 ⟨ O2+
So, the correct answer is “Option B”.
Note: You should notice that bond order is indirectly proportional to the length of the bond. The higher the bond order, the shorter and stronger will be the bond. The addition of each electron in the antibonding molecular orbital will decrease the bond order.
