Question
Question: Bond order of \[\text{B}{{\text{e}}_{\text{2}}}\] is A. 1 B. 2 C. 3 D. 0...
Bond order of Be2 is
A. 1
B. 2
C. 3
D. 0
Solution
According to the molecular orbital theory, the bond order is defined as the number of covalent bonds in a molecule. Bond order is equal to half of the difference between the number of electrons in bonding (Nb) and antibonding molecular orbitals (Na).
Complete Solution :
Be2 molecule will be formed by the overlapping of atomic orbitals of two beryllium atoms.
A Be atom has four electrons. It has two valence electrons and its electronic configuration is 1s22s2. Therefore, Be2 molecule has eight electrons which are to be filled in four molecular orbitals.
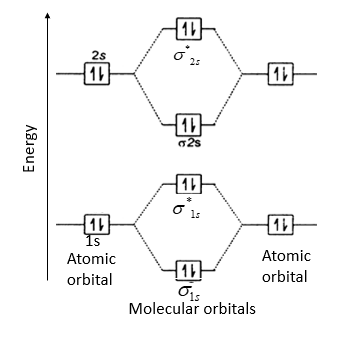
Thus, electronic configuration of Be2 is (σ1s)2(σ∗1s)2(σ2s)2(σ∗2s)2
Here, bonding electrons, Nb = 4 and anti-bonding electrons, Na= 4
Therefore, bond order (B.O.) of Be2 molecule is
