Question
Question: Bond order of N-O bond in \[NO_3^ - \] is: A. 1 B. 2 C. 3 D. 1.33...
Bond order of N-O bond in NO3− is:
A. 1
B. 2
C. 3
D. 1.33
Solution
In this question, the bond order of N-O in NO3− is calculated by dividing the number of chemical bonds present between the atoms of the ion divided by the total number of resonating structures.
Complete step by step answer:
The nitrate ion NO3−, can be represented by more than one structure known as resonance structure. The resonance structure is defined as the set of Lewis structure which describes the delocalization of electrons in a polyatomic ion.
The resonance structure of nitrate ion is shown below.
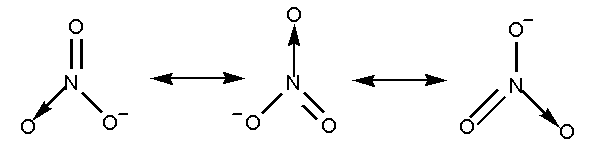
The nitrate ion contains three oxygen atoms attached to the nitrogen atom with a negative charge.
The bond order states the number of chemical bonds present between the atoms in a molecule. The bond order describes the stability of the bond.
For resonating structure, the bond order is calculated by the formula as shown below.
B.O=RB
Where,
B.O is the bond order
B is total number of chemical bond present
R is the total number of resonating structure
In nitrate ion two oxygen atoms are attached by a single bond and one oxygen atom is attached by a double bond. Total 4 bonds are present.
Total 3 resonating structures are formed by the nitrate ion.
Substitute the values in the above equation.
⇒B.O=34
⇒B.O=1.33
Thus, the bond order of N-O bonds in NO3− is 1.33.
Therefore, the correct option is D.
Note:
The bond order is usually calculated using molecular orbital theory where the number of bonding electrons is subtracted by the number of antibonding electrons divided by 2.
