Question
Question: $\bf{P}$ (C$_7$H$_{16}$) $\xrightarrow[\text{v) H}_3\text{O}^+]{\text{i) Cr}_2\text{O}_3\text{, 770 ...
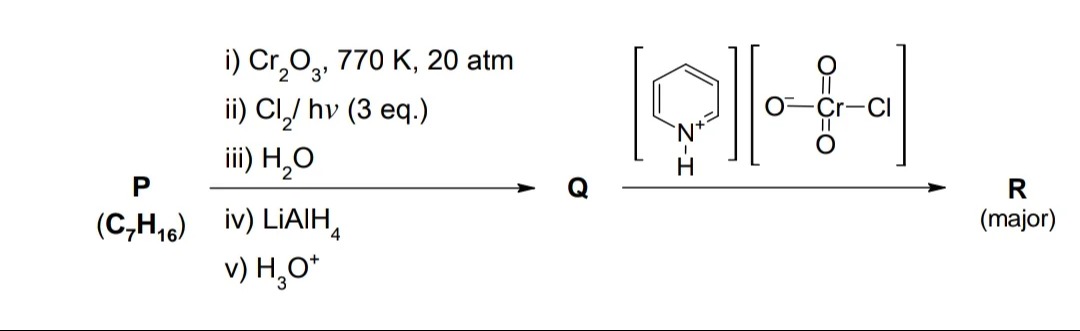
P (C7H16) i) Cr2O3, 770 K, 20 atmii) Cl2/ hv (3 eq.)iii) H2Oiv) LiAlH4v) H3O+ Q R (major)
Benzaldehyde
Solution
The reaction sequence starts with an alkane P with the formula C7H16.
Step i) P Cr2O3, 770 K, 20 atm Aromatization. This converts an alkane into an aromatic hydrocarbon. For a C7 alkane, the major aromatic product is toluene (methylbenzene), C7H8. This reaction involves cyclization and dehydrogenation. For example, n-heptane undergoes aromatization to toluene.
P (C7H16) → Toluene (C7H8)
Step ii) Toluene Cl2/ hv (3 eq.) Free radical halogenation at the benzylic position. With 3 equivalents of Cl2 and UV light, all three hydrogen atoms of the methyl group are substituted by chlorine atoms.
Toluene → Trichloromethylbenzene (C6H5CCl3)
Step iii) Trichloromethylbenzene H2O Hydrolysis of the geminal trichloride. This yields a carboxylic acid.
C6H5CCl3 + 3 H2O → C6H5C(OH)3 + 3 HCl → C6H5COOH + H2O
The product is benzoic acid (C6H5COOH).
Step iv) Benzoic acid LiAlH4 Reduction of the carboxylic acid to a primary alcohol.
C6H5COOH LiAlH4 C6H5CH2OH
The product is benzyl alcohol (C6H5CH2OH).
Step v) Benzyl alcohol H3O+ Acidic workup after LiAlH4 reduction. This step protonates the alkoxide intermediate to yield the alcohol. So, the product remains benzyl alcohol.
Thus, Q is benzyl alcohol (C6H5CH2OH).
The reaction from Q to R uses pyridinium chlorochromate (PCC), which is shown in the figure. PCC is a mild oxidizing agent that oxidizes primary alcohols to aldehydes and secondary alcohols to ketones. Benzyl alcohol is a primary alcohol.
Q (Benzyl alcohol) PCC R (major)
C6H5CH2OH PCC C6H5CHO
The major product R is benzaldehyde (C6H5CHO).
The reaction sequence involves aromatization of an alkane P to toluene, followed by free radical chlorination of toluene to trichloromethylbenzene. Hydrolysis of trichloromethylbenzene yields benzoic acid, which is then reduced by LiAlH4 to benzyl alcohol (Q). Finally, oxidation of benzyl alcohol (Q) with PCC gives benzaldehyde (R).
