Question
Question: Betamine occurs in the best sugar having molecular \( {C_5}{H_{11}}{O_2}N \) . Draw the structure of...
Betamine occurs in the best sugar having molecular C5H11O2N . Draw the structure of beta mine which is made by treatment of glycine with methyl iodine and which does not react with HCl .
Solution
Betamine is a chemical which occurs in the best sugar. The betamine molecule is having molecular formula C5H11O2N which is a three degree amine or tertiary amine, i.e, all the hydrogens are replaced with some functional groups or substituents.
Complete step-by-step answer:
First of all, we explore a little bit about betamine.
Betamine has an amine as functional group and it is a three degree amine or tertiary amine, i.e, all the hydrogens are replaced with some functional groups or substituents.
As given in the question, that betamine is made by treatment of glycine with methyl iodine and also the structure does not react with HCl .
Now, we will draw the structure of glycine and methyl iodide:
Glycine:
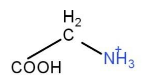
Methyl iodide:
CH3I
Now, we will proceed towards the reaction:
Reaction is as follows:
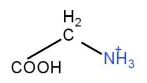 +CH3I→
+CH3I→ 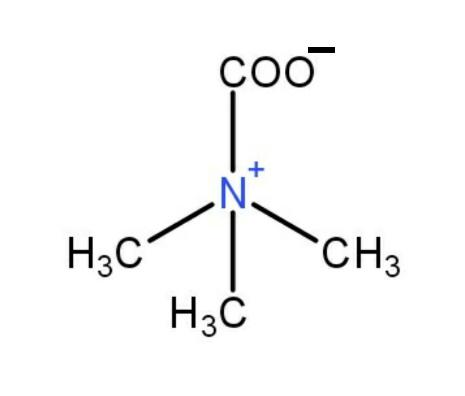
So, the structure is
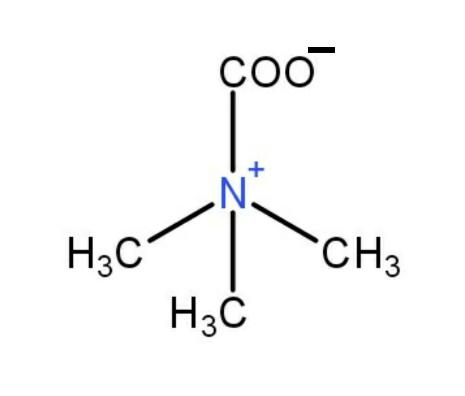
As given in question, the structure does not reacts with HCl :
Reaction with HCl :
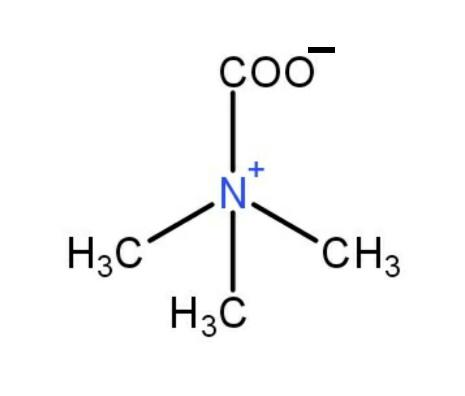 +HCl→ No reaction
+HCl→ No reaction
Also, there is no reaction with HCl , the structure is correct.
Hence, the structure of the betamine is
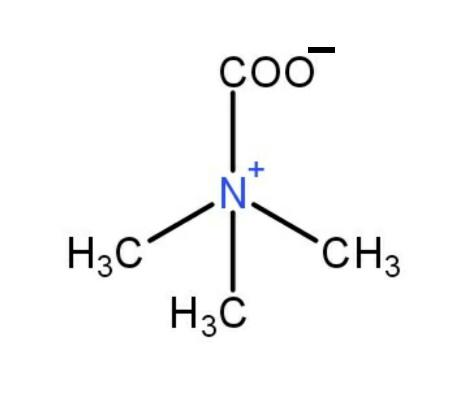 .
.
Additional Information:
The amines are of three types:
(a) Primary Amine: The ammonia in which only one hydrogen is replaced with other substituents, also known as one degree amine.
(b) Secondary Amine: The ammonia in which two hydrogens are replaced with other substituents, also known as two degree amine.
(c) Tertiary Amine: The ammonia in which all the three hydrogens are replaced with other substituents, also known as three degree amine.
Note:
Glycine is an amino acid, but it is not an essential amino acid because it can be made by itself within the body from the other chemicals already present in our body. Glycine is also a building block of the protein.
