Question
Question: Betaine \( {{\text{C}}_{\text{5}}}{{\text{H}}_{\text{11}}}{{\text{O}}_{\text{2}}}\text{N} \) occurs ...
Betaine C5H11O2N occurs in beet sugar molasses. It is water soluble that melts with decomposition at 300 0C . It is unaffected by a base but reacts with hydrochloric acid to form a crystalline product C5H12O2N Cl . It can be made from glycine with methyl iodide or treatment with chloroacetic acid with trimethyl amine. Draw the structure of betaine which will account for all the properties given above.
Solution
Hint:** Betaine is a neutral chemical compound with positively charged cationic functional group such the quaternary ammonium or the phosphonium ions that bears no hydrogen atom and a negatively charged functional group such as the carboxylate group. It is a zwitterion.
Complete stepwise solution:
When Betaine C5H11O2N reacts with hydrochloric acid to form a crystalline product C5H12O2N Cl , the reaction is shown as follows:
C5H11O2NHClC5H12O2NCl
The addition of hydrogen chloride to betiane to form the betiane hydrogen chloride salt indicates that betaine is a basic substance.
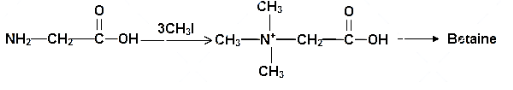
The above mechanism represents the mechanism for the formation of betiane from glycine. The glycine molecule is a small N-trimethylated amino acid. It is a zwitterion it cannot isomerize because there are no labile hydrogen atom attached to the nitrogen atom. This substance is called “glycine betiane” to distinguish it from the other betianes.
ClCH2COOH + N(CH3)3→ClCH2COOH.N(CH3)3
ClCH2COOH.N(CH3)3+NaI→ICH2COOH.N(CH3)3+NaCl
ICH2COOH.N(CH3)3+N(CH3)3→N(CH3)3CH2COO - +N(CH3)3.HI
The process of preparation of betaine from chloroacetic acid with triethylamine is an environmentally-friendly process to obtain high-purity betaine hydrochloride and the process consists of adding chloroacetic acid, a trimethylamine aqueous solution and Lewis base serving as a catalyst into a reaction vessel, reacting for 1 to 4 hours under the conditions that the temperature is 50 to 55 0C , and the pressure is 0.01 to 0.02 MPa .
Note:
In many naturally occurring systems, the betaines serve as organic “osmolytes”. These are substances taken up for the environment for the protection against osmotic stress, draught, high salinity, and high temperature. Intracellular accumulation of betaine allows water retention in cells.
