Question
Question: Benzyl chloride \(\left( {{C}_{6}}{{H}_{5}}C{{H}_{2}}Cl \right)\) can be prepared from toluene by ch...
Benzyl chloride (C6H5CH2Cl) can be prepared from toluene by chlorination with?
A. SO2Cl2
B. SOCl2
C. Cl2
D. NaOCI
Solution
Benzyl chloride(C6H5CH2Cl) can be prepared from toluene by chlorination with Cl2. This is because it is found that in the presence of sunlight, Cl2 gives free radicals. This reaction is basically a free radical substitution reaction.
Complete answer:
- We can see that in toluene there is no electrophilic centre, hence oxidation takes place in the presence of sunlight using Cl2.
- SO2Cl2 is found to give chlorination, when there is a nucleophilic substitution reaction on an electrophilic centre. But as we know, there is no electrophilic centre in toluene, therefore, chlorination occurs by a controlled free radical mechanism.
- NaOCI Is found to give substitution reaction, and doesn’t give free radical so it will take part in chlorination.
- We can see the chlorination reaction that is, toluene by chlorination with Cl2 will give Benzyl chloride as:
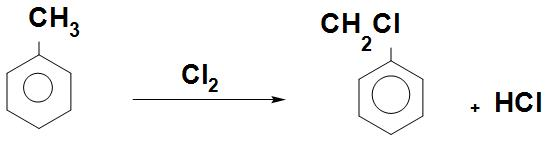
- Benzyl chloride is used in large scale in making resins, dyes, lubricants, drugs and cosmetics.
- Hence, we can conclude that the correct option is (c), that is Benzyl chloride(C6H5CH2Cl) can be prepared from toluene by chlorination with Cl2.
Note: - Industrially, Benzyl chloride can be prepared from toluene and chlorine by the gas phase photochemical mechanism. During this reaction HCl is obtained as a side product.
- It is also found that Benzyl chloride has a strong smell, inflammable to skin and can cause tearing of eyes.
