Question
Question: Benzyl chloride \({C_6}{H_5}C{H_2}Cl\) can be prepared from toluene by chlorination with : A. \(C{...
Benzyl chloride C6H5CH2Cl can be prepared from toluene by chlorination with :
A. Cl2
B. SO2Cl2
C. SOCl2
D. NaOCl
Solution
Hint: In methods of preparation of aromatic halides we have studied that benzyl chloride can be formed from toluene by chlorination with Cl2 because chlorine can give free radical in presence of sunlight. This reaction of chlorination is a free radical substitution reaction.
Complete step by step solution:
Here toluene does not have any electrophilic centre so oxidation occurs by controlled free radical using Cl2 in presence of sunlight. SOCl2 gives chlorination when it takes part in nucleophilic substitution on an electrophilic carbon. In toluene there is no electrophilic centre so chlorination occurs by controlled free radical reaction using chlorine. Here in these options NaOCl gives substitution reaction it does not give free radical so it will not take part in chlorination. Benzyl chloride or C6H5CH2Cl can be prepared from toluene by chlorination with Cl2 . It is equilateral to photochemical reactions. The reaction of chlorination is given below: 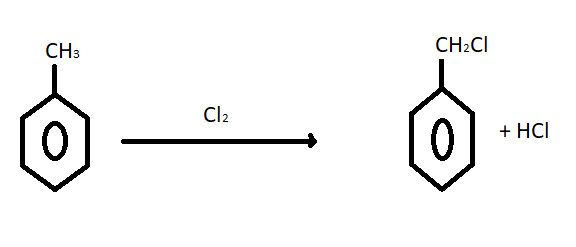 Benzyl chloride is used in making dyes, drugs, resins, lubricants, plasticizer and cosmetics. It is a colourless liquid with a strong smell and can cause tearing of eyes. It is very inflammable to skin. Benzoic acid can be prepared from oxidation of benzyl chloride. Hence option A is the correct answer to this problem. Benzyl chloride C6H5CH2Cl could be prepared from toluene by chlorination with Cl2.
Benzyl chloride is used in making dyes, drugs, resins, lubricants, plasticizer and cosmetics. It is a colourless liquid with a strong smell and can cause tearing of eyes. It is very inflammable to skin. Benzoic acid can be prepared from oxidation of benzyl chloride. Hence option A is the correct answer to this problem. Benzyl chloride C6H5CH2Cl could be prepared from toluene by chlorination with Cl2.
Note: Benzyl chloride is used to be prepared industrially by the gas phase photochemical reaction of toluene with chlorine. This reaction proceeds by free radical mechanism involving the free chlorine intermediate. Side product is hydrochloric acid. So in the given options only Cl2 can give chloride free radical in the presence of sunlight.
