Question
Question: Benzoylation of phenol in alkaline medium is known as A) Friedel-Craft reaction B) Wurtz-Fittig...
Benzoylation of phenol in alkaline medium is known as
A) Friedel-Craft reaction
B) Wurtz-Fittig reaction
C) Schotten-Baumann reaction
D) Sabatier Senderens reductions
Solution
As we know that in organic chemistry there are so many naming reactions. Phenol is one of the aromatic compounds. Phenol is nothing but a hydroxyl group replaced with one hydrogen in benzene ring. The substitution reaction means one group is substituted by another group in the organic compound. We need to know that the benzoylation means by benzoyl chloride is the most electronegative hydrogen in reactant is replaced by benzoyl group to form a product.
Complete step by step answer:
We need to know that when phenol reacts with Benzoylation, hydrogen in the hydroxyl group is replaced with the benzoyl group to form phenyl benzoate.
We must have to know that the Friedel-craft reaction means anhydrous aluminium chloride acts as catalyst for acylation of benzene moiety. But not used as an alkaline medium for the reaction. We can write the chemical equation for this chemical reaction as,
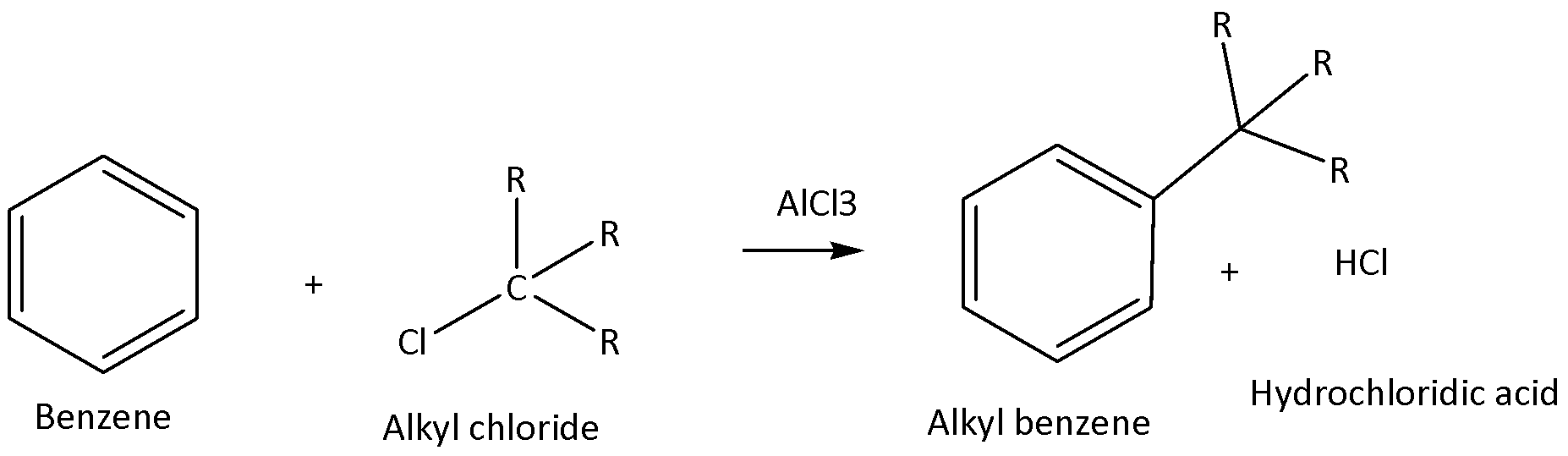
Therefore, option A is not the correct answer.
We have to remember that the Wurtz-fittig reaction means using sodium metal and alkyl halides by dry ether as solvent, aryl halide to aromatic compounds. Like benzyl chloride it reacts with methyl chloride in presence of sodium and dry ether as solvent to form a product of methyl benzene. We can write the chemical equation for this chemical reaction as,
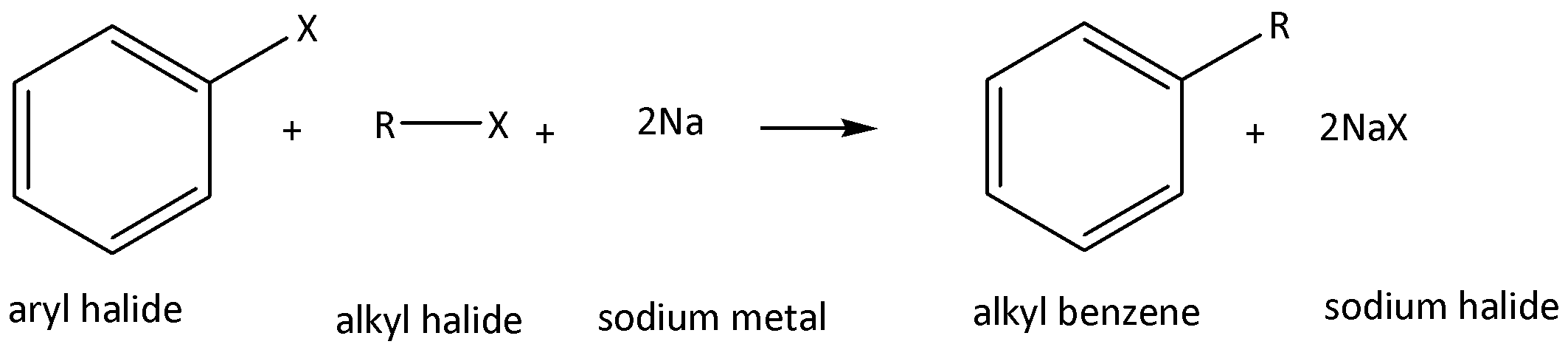
Therefore, option B is not the correct answer.
We need to know that the Sabatier Senderens reductions means hydrogen reacts with carbon dioxide in temperature range 300∘C−400∘C and 30 bar pressure to form methane and water. Ruthenium is a suitable catalyst for this reaction on alumina oxide to enhance its reactivity. We can write the chemical equation for this chemical reaction as,
CO2+4H2→CH4+2H2O
We must have to remember that the Schotten-Baumann reaction means phenol reacts with benzyl chloride in presence of alkaline medium (sodium hydroxide) to form phenyl benzoate with removal of hydrochloride. We can write the chemical equation for this chemical reaction as,
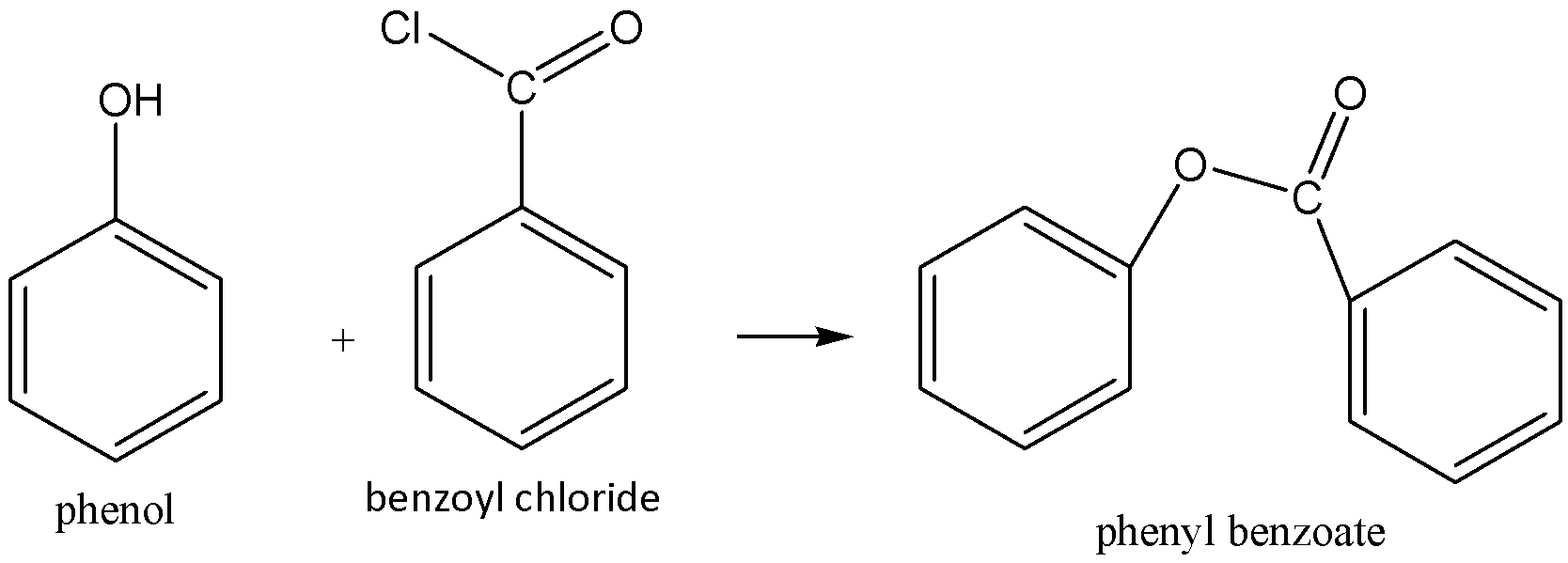
Benzoylation of phenol in the alkaline medium is known as Schotten-Baumann reaction.
Therefore, option c is correct.
Note:
We need to remember that the benzoylation of any benzene moiety having at least one hydrogen atom bonded to electronegativity atom. Benzoylation in benzene compared to another benzene derivative is less reactive. Because all the hydrogen is having the same electronegativity. Benzoylation is an important reaction for forming larger benzene moieties. In new drug designing purposes, most of the scientists use these techniques for synthesis of new molecules. In this reaction, larger benzene moiety is also applicable.
