Question
Question: Benzoyl chloride is prepared from benzoic acid by: A. \(C{l_2}\) and \(h\nu \) B. \(S{O_2}C{l_2}...
Benzoyl chloride is prepared from benzoic acid by:
A. Cl2 and hν
B. SO2Cl2
C. SOCl2
D. Cl2 and H2O
Solution
Benzoyl chloride has molecular formula C6H5COCl with structure
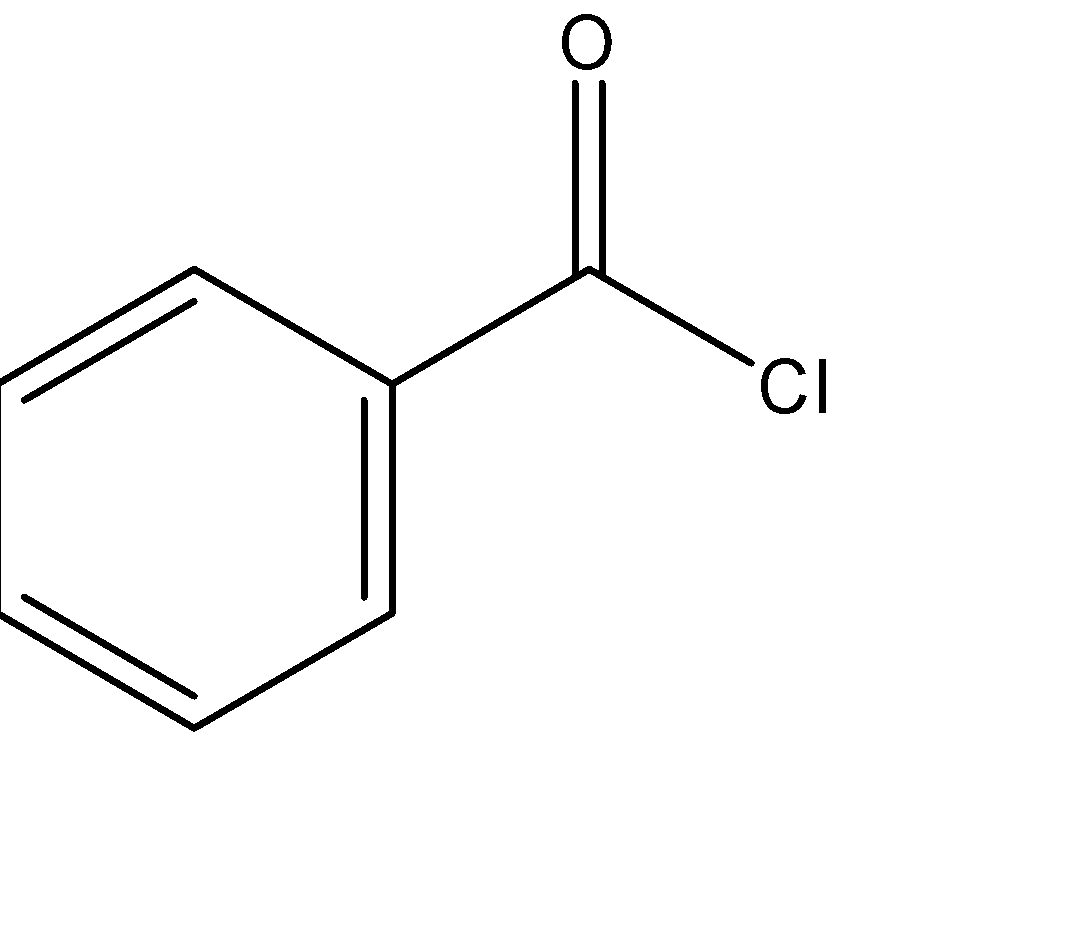
Benzoic acid is C6H5COOH
Benzoyl chloride can be prepared from benzoic acid. Here we can see that the OH group in benzoic acid is replaced by Cl in benzoyl chloride. Therefore a Chlorine compound is added.
Complete step by step answer:
Option A is a method of addition of chlorine atom to a compound. But it is not applicable here as there is no alkane molecule here. If it is present then, Cl2 and hν can act as a chlorinating agent. If there is CH3−CH2−here, Cl2 and hν can add a chlorine atom.
Here, benzoyl chloride can be obtained by treated benzoic acid with SOCl2. Here, SO2+HCl is removed. SOCl2 is also called thionyl chloride. Thionyl chloride readily replaces OH to Cl.
Benzoic acid does not react with Cl2 and H2O
PhCOOHSOCl2PhCOCl+SO2+HCl
So, the correct answer is Option C.
Note: There are many other methods of preparing benzoyl chloride. The main use of thionyl chloride is as a chlorinating agent.
Benzoyl chloride can also be prepared from benzoic acid by treating it with PCl5.
Similarly, if the benzoic acid is treated with benzyl trichloride, benzoyl chloride can be obtained.
Thus, benzoic acid can be used to prepare benzoyl chloride by treating it with phosphorus pentachloride, thionyl chloride or benzyl trichloride.
