Question
Question: Benzoic acid on treatment with hydrazoic acid \(\left( {{{\text{N}}_{\text{3}}}{\text{H}}} \right)\)...
Benzoic acid on treatment with hydrazoic acid (N3H) in the presence of concentrated sulphuric acid gives:
A. Benzamide
B. Sodium benzoate
C. Aniline
D. C6H5CON3
Solution
In the above question, it is asked what happens when benzoic acid reacts with hydrazoic acid (N3H) in the presence of concentrated sulphuric acid. This is an example of Schmidt reaction. It results in formation of an amine.
Complete step-by-step answer: The Schmidt reaction is an organic reaction in which an azide reacts with a carbonyl derivative, usually an aldehyde, ketone, or carboxylic acid, under acidic conditions to give an amine, imide or amide.
When carboxylic acid undergoes Schmidt reaction it results in formation of amine with one carbon less.
The mechanism of formation of amine involves formation of acylium ion, which is followed by protonated azido ketone and then protonated isocyanate ion is formed and finally hydrolysis yields amine and CO2.
So, when benzoic acid undergoes Schmidt reaction, aniline is formed.
The reaction involving the formation of aniline from benzoic acid is given below:
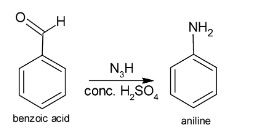
Additional information:
Benzoic acids are used in the production of phenol as well as ointments that prevent fungal disease. It is used in cosmetic products such as lipsticks. It is also used in manufacture of dyes and insect repellants.
Aniline is used as dyeing agent in the manufacture of clothes like jeans. It is employed in production of drugs. It is also used as pesticides and fungicides.
So, the correct option is option C.
Note: When ketone undergoes Schmidt reaction, amide is formed.
When alkene undergoes Schmidt reaction, imine is formed.
When carboxylic acid undergoes Schmidt reaction, amine is formed.
