Question
Question: Benzene with molecular formula \({C_6}{H_6}\) has: Option: A.\(6\) Single bond and \(6\) double ...
Benzene with molecular formula C6H6 has:
Option:
A.6 Single bond and 6 double bond
B.12 Single bond and 3 double bond
C.18 Single bond only
D.12 double bond only
Solution
Benzene is defined as an aromatic compound which is composed of only carbon and hydrogen atoms therefore it is also known as aromatic hydrocarbon. Structure of benzene was successfully described by Kekule.
Complete answer:
As we know, the molecular formula of benzene is C6H6. According to the basic concept of orbital theory, all the 6 carbon atoms of benzene are sp2 hybridized. When a carbon atom undergoes overlapping, out of three hybrid orbital of the carbon atom two undergoes axillary overlapping with the adjacent carbon atoms to form the bond. While the remaining last hybrid orbital of carbon undergoes overlapping with the hydrogen atom to form a bond with it.
From the above discussion we finally draw the structure of benzene:
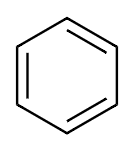
All the carbon atoms of benzene attached to one hydrogen atom to complete its valency to 4. So the final structure of benzene will be:
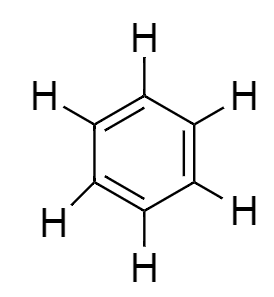
From the above diagram we easily calculate that there are 6 single bonds formed between all the six-carbon atoms along with 6single bonds with hydrogen atoms. So, there are a total number of 12 single bonds in a single benzene.
Now we see that there are 3 more bonds present in structure between carbon atoms. These are double bonds formed between adjacent carbon atoms of benzene.
⇒ There are 12 Single bonds and 3 double bonds that are present in benzene molecules.
Therefore, option (B) is the correct option.
Note:
Benzene possesses a very distinctive odour which helps in identification of it. Benzene is unsaturated in nature due to presence of 3 double bonds but still it does not participate in decolorization reaction with bromine in CCl4 and KMnO4 alkaline solution.
