Question
Question: Benzene reacts with \(C{H_3}Cl\) in the presence of anhydrous \(AlC{l_3}\) to form: A. Xylene B....
Benzene reacts with CH3Cl in the presence of anhydrous AlCl3 to form:
A. Xylene
B. toluene
C. Chlorobenzene
D. benzyl chloride
Solution
A Friedel-Crafts reaction is a type of organic coupling reaction that uses an electrophilic aromatic substitution to attach substituents to aromatic rings. Alkylation and acylation reactions are the two most common Friedel-Crafts reactions.
Complete answer:
Benzene reacts with CH3Cl in the presence of anhydrous AlCl3 to give toluene.
CH3Cl in the presence of anhydrous AlCl3 acts as an alkylation agent and introduces an alkyl group. This reaction is known as Friedel- Crafts alkylation of benzene.
The reaction is as follows:
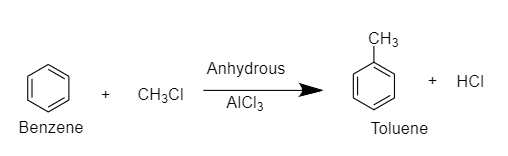
Friedel-Crafts Alkylation refers to the substitution of an alkyl group for an aromatic proton. With the help of a carbocation, an electrophilic attack on the aromatic ring is carried out. The Friedel-Crafts alkylation reaction uses alkyl halides as reactants to produce alkylbenzenes.
Limitations of Friedel Crafts Alkylation
The Friedel-Crafts acylation reaction has a few drawbacks, while resolving some of the constraints of the related alkylation reaction (such as carbocation rearrangement and polyalkylation).
Ketones are the only products of the acylation process. When exposed to these conditions, formyl chloride H(C=O)Cl decomposes into CO and HCl.
If the aromatic compound is less reactive than a mono-halobenzene, it cannot participate in this reaction.
Because aryl amines form very unreactive complexes with the Lewis acid catalyst, they cannot be employed in this reaction.
Hence, the correct option is B. toluene.
Note:
It should be noted that in Friedel Craft ‘s reactions, a hydrogen atom (which was originally linked to the aromatic ring) is replaced with an electrophile. Since it works as a Lewis acid and coordinates with the halogens, forming an electrophile in the process, aluminium trichloride (AlCl3) is frequently used as a catalyst in Friedel-Crafts processes.
