Question
Question: Benzene is passed through \[Na\] in \[N{H_3}\] solution then the major product was passed through oz...
Benzene is passed through Na in NH3 solution then the major product was passed through ozone and Zn−H2O solution then the product of it was passed through Zn−Hg in Conc.HCl solution to give a product. What and how is the product formed?
Solution
Benzene is an aromatic compound and when reacted with sodium in ammonia undergo reduction to form 1,4 cyclohexadiene. When this compound passes through ozone and Zn−H2O solution forms an aldehyde compound. When aldehyde is passed through Zn−Hg in Conc.HCl solution forms propane.
Complete answer:
Given that benzene is passed through Na in NH3 solution. The reaction is named as Birch reduction. Aromatic compound when passed through Na in NH3 solution undergoes reduction to form a 1,4 cyclohexadiene.
The chemical reaction involved will be as follows-
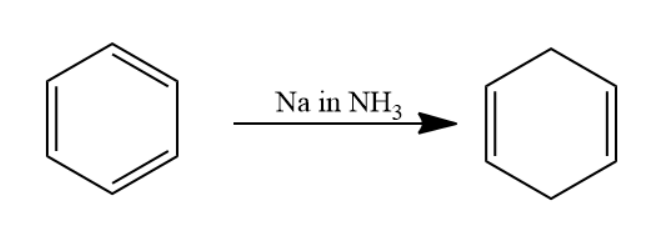
When this 1,4 cyclohexadiene is passed through ozone and Zn−H2O solution the double bond will converts into carbonyl group forms two molecules of dialdehyde compound named as 1,3−propane−di−al. Two moles of dialdehyde compound form in this reaction.
The chemical reaction involved will be as follows-
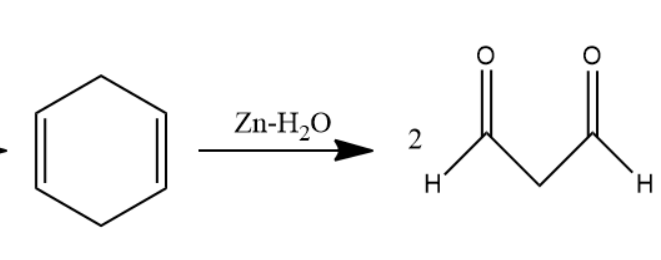
Later, 1,3−propane−di−al passed through Zn−Hg in Conc.HCl solution undergoes clemmensen reduction to form alkanes. Carbonyl group converts into alkane in clemmensen reduction. Two molecules of 1,3−propane−di−al convert into two molecules of propane.
The chemical reaction involved will be as follows:
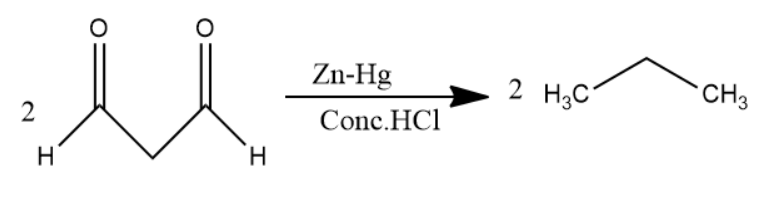
Thus, Benzene is passed through Na in NH3 solution then the major product was passed through ozone and Zn−H2O solution then the product of it was passed through Zn−Hg in Conc.HCl solution to give a product. The product is propane.
Note:
Birch reduction can occur in two types based on the group present on the aromatic ring. When there is a presence of an electron releasing group then the reduction takes place at 2,5 positions. When there is a presence of an electron withdrawing group then the reduction takes place at 1,4 positions.
